Decreased Na(+) influx lowers hippocampal neuronal excitability in a mouse model of neonatal influenza infection
- PMID: 26310542
- PMCID: PMC4550875
- DOI: 10.1038/srep13440
Decreased Na(+) influx lowers hippocampal neuronal excitability in a mouse model of neonatal influenza infection
Abstract
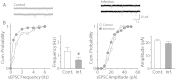
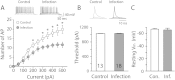
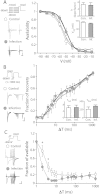
Influenza virus infection is one of common infectious diseases occurring worldwide. The human influenza virus can infect the central nervous system and cause brain dysfunctions affecting cognition and spatial memory. It has been previously shown that infection with the influenza viral protein within the hippocampus decreases Ca(2+) influx and reduces excitatory postsynaptic currents. However, the neuronal properties of animals surviving neonatal infection have not been investigated. Using a mouse model of neonatal influenza infection, we performed thorough electrophysiological analyses of hippocampal neurotransmission. We found that animals surviving the infection exhibited reduced spontaneous transmission with no significant defects in evoked neurotransmission. Interestingly, the hippocampus of the infected group conducted synaptic transmission with less fidelity upon repeated stimulations and failed to generate action potentials faithfully upon step current injections primarily due to reduced Na(+) influx. The reversal potential for the Na(+) current was hyperpolarized and the activation of Na(+) channels was slower in the infected group while the inactivation process was minimally disturbed. Taken together, our observations suggest that neonatally infected offsprings exhibit noticeable deficits at rest and severe failures when higher activity is required. This study provides insight into understanding the cellular mechanisms of influenza infection-associated functional changes in the brain.
Figures



Similar articles
-
Neonatal influenza virus infection affects myelination in influenza-recovered mouse brain.J Vet Sci. 2018 Nov 30;19(6):750-758. doi: 10.4142/jvs.2018.19.6.750. J Vet Sci. 2018. PMID: 30173495 Free PMC article.
-
Changes in calcium currents and GABAergic spontaneous activity in cultured rat hippocampal neurons after a neurotropic influenza A virus infection.Brain Res Bull. 2001 Jun;55(3):421-9. doi: 10.1016/s0361-9230(01)00536-6. Brain Res Bull. 2001. PMID: 11489350
-
Involvement of Potassium and Cation Channels in Hippocampal Abnormalities of Embryonic Ts65Dn and Tc1 Trisomic Mice.EBioMedicine. 2015 Jul 31;2(9):1048-62. doi: 10.1016/j.ebiom.2015.07.038. eCollection 2015 Sep. EBioMedicine. 2015. PMID: 26501103 Free PMC article.
-
Neurophysiology of HCN channels: from cellular functions to multiple regulations.Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
-
[Mechanism of action of neurotoxins acting on the inactivation of voltage-gated sodium channels].C R Seances Soc Biol Fil. 1998;192(3):409-36. C R Seances Soc Biol Fil. 1998. PMID: 9759381 Review. French.
Cited by
-
Neonatal influenza virus infection affects myelination in influenza-recovered mouse brain.J Vet Sci. 2018 Nov 30;19(6):750-758. doi: 10.4142/jvs.2018.19.6.750. J Vet Sci. 2018. PMID: 30173495 Free PMC article.
-
Neuroinvasive and neurovirulent potential of SARS-CoV-2 in the acute and post-acute phase of intranasally inoculated ferrets.PLoS One. 2025 Apr 7;20(4):e0311449. doi: 10.1371/journal.pone.0311449. eCollection 2025. PLoS One. 2025. PMID: 40193353 Free PMC article.
References
-
- Kristensson K. Avian influenza and the brain–comments on the occasion of resurrection of the Spanish flu virus. Brain Res Bull. 68, 406–413 (2006). - PubMed
-
- Ekstrand J. J. Neurologic complications of influenza. Semin Pediatr Neurol. 19, 96–100 (2012). - PubMed
-
- Baltagi S. A., Shoykhet M., Felmet K., Kochanek P. M. & Bell M. J. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 11, 179–184 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

