The Trp53 delta proline (Trp53ΔP) mouse exhibits increased genome instability and susceptibility to radiation-induced, but not spontaneous, tumor development
- PMID: 26310697
- PMCID: PMC4891300
- DOI: 10.1002/mc.22377
The Trp53 delta proline (Trp53ΔP) mouse exhibits increased genome instability and susceptibility to radiation-induced, but not spontaneous, tumor development
Abstract
The tumor suppressor TP53 can initiate a plethora of anti-proliferative effects to maintain genomic integrity under conditions of genotoxic stress. The N-terminal proline-rich domain (PRD) of TP53 is important in the regulation of TP53 activity and stability. A common polymorphism at codon 72 in this region has been associated with altered cancer risk in humans. The Trp53ΔP mouse, which carries a germline homozygous deletion of a region of the PRD, does not develop spontaneous tumors in a mixed 129/Sv and C57BL/6 genetic background, but is highly susceptible to a broad range of tumor types following total body exposure to 4 Gy gamma (γ) radiation. This contrasts with the tumor spectrum in Trp53 null (-/-) mice, which mainly develop thymic lymphomas and osteosarcomas. Analysis of genomic instability in tissues and cells from Trp53ΔP mice demonstrated elevated basal levels of aneuploidy, but this is not sufficient to drive spontaneous tumorigenesis, which requires an additional DNA damage stimulus. Levels of genomic instability did not increase significantly in Trp53ΔP mice following irradiation exposure, suggesting that other radiation effects including tissue inflammation, altered metabolism or autophagy, may play an important role. The Trp53ΔP mouse is a novel model to dissect the mechanisms of tumor development induced by radiation exposure. © 2015 Wiley Periodicals, Inc.
Keywords: Trp53; cancer radiation; genomic instability.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures

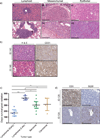

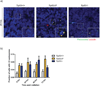
References
-
- Marx J. Debate surges over the origins of genomic defects in cancer. Science. 2002;297:544–546. - PubMed
-
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. - PubMed
-
- Lane D. p53, guardian of the genome. Nature. 1992;358:15–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

