Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma
- PMID: 26310711
- PMCID: PMC4714676
- DOI: 10.1111/cas.12800
Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma
Abstract
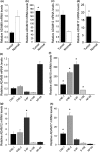
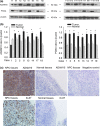
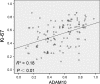
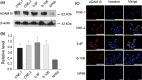
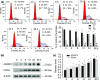
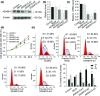
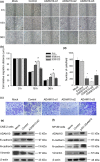
A disintegrin and metalloprotease 10 (ADAM10) is a typical member of the ADAMs family, which has been reported to be upregulated in various types of cancers and contribute to cancer progression and metastasis. However, little is known about the role of ADAM10 in nasopharyngeal carcinoma (NPC). The purpose of this study is to explore ADAM10 expression status and its biological functions in NPC. We first examined the expression of ADAM10 in NPC tissues and cell lines by immunohistochemistry, Western blotting, PCR, and immunofluorescence analysis. We observed that ADAM10 was significantly elevated in NPC and its expression level was correlated with T classification (P = 0.044), distant metastasis (P = 0.016), TNM clinical stage (P = 0.013), and proliferation marker Ki-67 expression (P = 0.001). Patients with NPC with high expression of ADAM10 had shorter overall survival rates. In addition, knockdown of ADAM10 by RNAi was found to inhibit the CNE-2 cell proliferation and migration. Our findings hinted that overexpression of ADAM10 promotes the progression and migration of NPC, which makes it a potential therapeutic target for the treatment of tumor metastases in NPC.
Keywords: ADAM10; migration; nasopharyngeal carcinoma; prognosis; progression.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
ADAM10 regulates proliferation, invasion, and chemoresistance of bladder cancer cells.Tumour Biol. 2014 Sep;35(9):9263-8. doi: 10.1007/s13277-014-2201-9. Epub 2014 Jun 18. Tumour Biol. 2014. PMID: 24935471
-
ADAM10 is overexpressed in human hepatocellular carcinoma and contributes to the proliferation, invasion and migration of HepG2 cells.Oncol Rep. 2013 Oct;30(4):1715-22. doi: 10.3892/or.2013.2650. Epub 2013 Aug 2. Oncol Rep. 2013. PMID: 23912592
-
Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells.Tumour Biol. 2016 Jun;37(6):8169-79. doi: 10.1007/s13277-015-4686-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715277
-
Matrix Metalloproteinase 14 Overexpression Is Correlated with the Progression and Poor Prognosis of Nasopharyngeal Carcinoma.Arch Med Res. 2015 Apr;46(3):186-92. doi: 10.1016/j.arcmed.2015.03.006. Epub 2015 Mar 28. Arch Med Res. 2015. PMID: 25829357
-
Prognostic value of TROP2 in human nasopharyngeal carcinoma.Int J Clin Exp Pathol. 2015 Sep 1;8(9):10995-1004. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617817 Free PMC article.
Cited by
-
MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma.Autophagy. 2021 Jul;17(7):1667-1683. doi: 10.1080/15548627.2020.1781368. Epub 2020 Jul 5. Autophagy. 2021. PMID: 32627648 Free PMC article.
-
Efficacy of concurrent chemoradiotherapy combined with nimotuzumab in the treatment of nasopharyngeal carcinoma with cervical lymph node metastasis.Eur Arch Otorhinolaryngol. 2023 May;280(5):2479-2488. doi: 10.1007/s00405-022-07805-w. Epub 2022 Dec 29. Eur Arch Otorhinolaryngol. 2023. PMID: 36577788
-
Reduced expression of polymeric immunoglobulin receptor (pIgR) in nasopharyngeal carcinoma and its correlation with prognosis.Tumour Biol. 2016 Aug;37(8):11099-104. doi: 10.1007/s13277-016-4791-x. Epub 2016 Feb 24. Tumour Biol. 2016. PMID: 26910773
-
ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance.Oncoimmunology. 2020 Apr 14;9(1):1744980. doi: 10.1080/2162402X.2020.1744980. eCollection 2020. Oncoimmunology. 2020. PMID: 32363112 Free PMC article.
-
Overexpression of A disintegrin and metalloprotease 10 promotes tumor proliferation, migration and poor prognosis in hypopharyngeal squamous cell carcinoma.Oncol Rep. 2017 Aug;38(2):866-874. doi: 10.3892/or.2017.5761. Epub 2017 Jun 27. Oncol Rep. 2017. PMID: 28656294 Free PMC article.
References
-
- Wei KR, Yu YL, Yang YY et al Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev 2010; 11 (1): 29–32. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55 (2): 74–108. - PubMed
-
- Lin YT, Wang LF, Hsu YC. Curcuminoids suppress the growth of pharynx and nasopharyngeal carcinoma cells through induced apoptosis. J Agric Food Chem 2009; 57: 3765–70. - PubMed
-
- Chan AT, Leung SF, Ngan RK et al Overall survival after concurrent cisplatin‐radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005; 97: 536–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

