Advances in molecular-based personalized non-small-cell lung cancer therapy: targeting epidermal growth factor receptor and mechanisms of resistance
- PMID: 26310719
- PMCID: PMC4673988
- DOI: 10.1002/cam4.506
Advances in molecular-based personalized non-small-cell lung cancer therapy: targeting epidermal growth factor receptor and mechanisms of resistance
Abstract
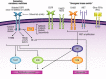
Molecularly targeted therapies, directed against the features of a given tumor, have allowed for a personalized approach to the treatment of advanced non-small-cell lung cancer (NSCLC). The reversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib had undergone turbulent clinical development until it was discovered that these agents have preferential activity in patients with NSCLC harboring activating EGFR mutations. Since then, a number of phase 3 clinical trials have collectively shown that EGFR-TKI monotherapy is more effective than combination chemotherapy as first-line therapy for EGFR mutation-positive advanced NSCLC. The next generation of EGFR-directed agents for EGFR mutation-positive advanced NSCLC is irreversible TKIs against EGFR and other ErbB family members, including afatinib, which was recently approved, and dacomitinib, which is currently being tested in phase 3 trials. As research efforts continue to explore the various proposed mechanisms of acquired resistance to EGFR-TKI therapy, agents that target signaling pathways downstream of EGFR are being studied in combination with EGFR TKIs in molecularly selected advanced NSCLC. Overall, the results of numerous ongoing phase 3 trials involving the EGFR TKIs will be instrumental in determining whether further gains in personalized therapy for advanced NSCLC are attainable with newer agents and combinations. This article reviews key clinical trial data for personalized NSCLC therapy with agents that target the EGFR and related pathways, specifically based on molecular characteristics of individual tumors, and mechanisms of resistance.
Keywords: Afatinib; dacomitinib; erlotinib; gefitinib; non-small-cell lung cancer; resistance.
© 2015 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- National Comprehensive Cancer Network. 2015. NCCN clinical practice guideline in oncology. Non-Small Cell Lung Cancer. Version 5.2015.
-
- Iressa. Iressa®. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2005. (gefitinib tablets) [package insert]
-
- Avastin. AVASTIN®. South San Francisco, CA: Genentech, Inc; 2014. (bevacizumab). Solution for intravenous infusion [package insert]
-
- Tarceva. TARCEVA®. South San Francisco, CA: Genentech, Inc; 2014. (erlotinib) tablets, for oral use [package insert]
-
- XALKORI. XALKORI®. New York, NY: Pfizer Labs; 2014. (crizotinib) capsules, oral [package insert]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

