Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds
- PMID: 26310728
- PMCID: PMC4550933
- DOI: 10.1038/srep13515
Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds
Abstract
Magnetically recoverable noble metal nanoparticles are promising catalysts for chemical reactions. However, the chemical synthesis of these nanocatalysts generally causes environmental concern due to usage of toxic chemicals under extreme conditions. Here, Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites are biosynthesized under ambient and physiological conditions by Shewanella oneidensis MR-1. Microbial cells firstly transform akaganeite into magnetite, which then serves as support for the further synthesis of Pd, Au and PdAu nanoparticles from respective precursor salts. Surface-bound cellular components and exopolysaccharides not only function as shape-directing agent to convert some Fe3O4 nanoparticles to nanorods, but also participate in the formation of PdAu alloy nanoparticles on magnetite. All these three kinds of magnetic nanocomposites can catalyze the reduction of 4-nitrophenol and some other nitroaromatic compounds by NaBH4. PdAu/Fe3O4 demonstrates higher catalytic activity than Pd/Fe3O4 and Au/Fe3O4. Moreover, the magnetic nanocomposites can be easily recovered through magnetic decantation after catalysis reaction. PdAu/Fe3O4 can be reused in at least eight successive cycles of 4-nitrophenol reduction. The biosynthesis approach presented here does not require harmful agents or rigorous conditions and thus provides facile and environmentally benign choice for the preparation of magnetic noble metal nanocatalysts.
Figures

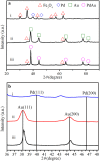
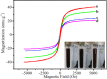
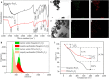

References
-
- Moreno M., Ibanez F. J., Jasinski J. B. & Zamborini F. P. Hydrogen reactivity of palladium nanoparticles coated with mixed monolayers of alkyl thiols and alkyl amines for sensing and catalysis applications. J. Am. Chem. Soc. 133, 4389–4397 (2011). - PubMed
-
- Chen H., Wei G., Ispas A., Hickey S. G. & Eychmüller A. Synthesis of palladium nanoparticles and their applications for surface-enhanced Raman scattering and electrocatalysis. J. Phys. Chem. C 114, 21976–21981 (2010).
-
- Fashedemi O. O., Julies B. & Ozoemena K. I. Synthesis of Pd-coated FeCo@ Fe/C core–shell nanoparticles: microwave-induced ‘top-down’ nanostructuring and decoration. Chem. Commun. 49, 2034–2036 (2013). - PubMed
-
- Xi P. et al. Surfactant free RGO/Pd nanocomposites as highly active heterogeneous catalysts for the hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. Nanoscale 4, 5597–5601 (2012). - PubMed
-
- Lloyd J. R., Byrne J. M. & Coker V. S. Biotechnological synthesis of functional nanomaterials. Curr. Opin. Biotech. 22, 509–515 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

