Increased exposure to acute thermal stress is associated with a non-linear increase in recombination frequency and an independent linear decrease in fitness in Drosophila
- PMID: 26310872
- PMCID: PMC4551699
- DOI: 10.1186/s12862-015-0452-8
Increased exposure to acute thermal stress is associated with a non-linear increase in recombination frequency and an independent linear decrease in fitness in Drosophila
Abstract
Background: Meiotic recombination rate has long been known to be phenotypically plastic. How plastic recombination evolves and is maintained remains controversial; though a leading model for the evolution of plastic recombination rests on the tenet that organismal fitness and recombination frequency are negatively correlated. Motivated by the mounting evidence that meiotic recombination frequencies increase in response to stress, here we test for a negative correlation between fitness and recombination frequency. Specifically, the fitness-associated recombination model (FAR) predicts that if stress increases meiotic recombination frequency, then increasing exposure to stressful conditions will yield an increasing magnitude of the recombinational response, while concomitantly decreasing fitness.
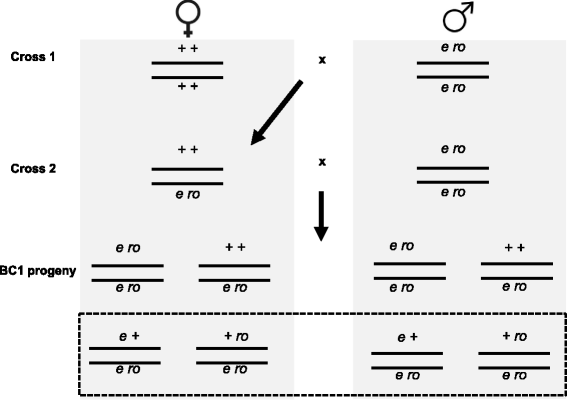
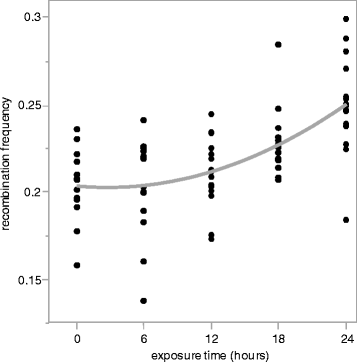
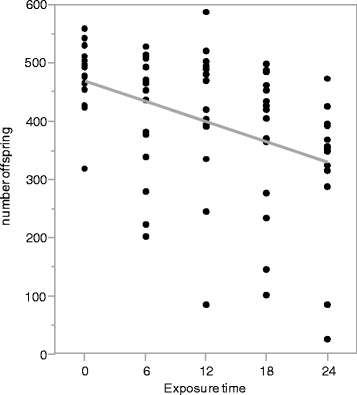
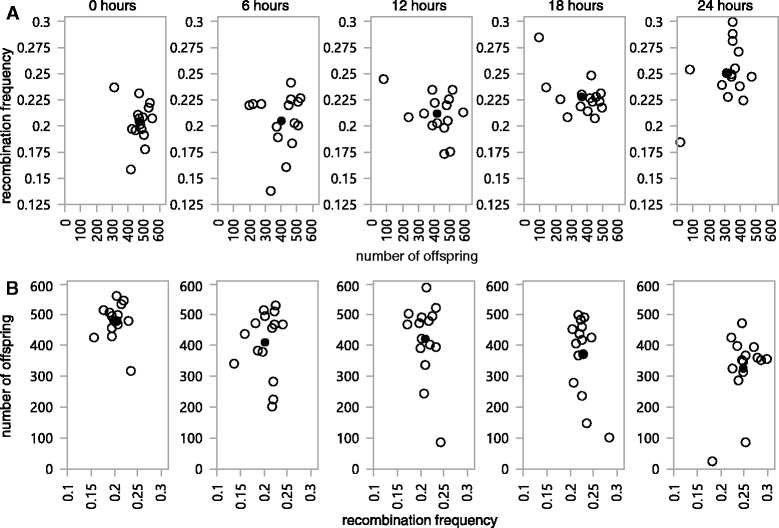
Results: We use heat shock as a stressor to test this prediction in Drosophila melanogaster. We find that increased exposure to heat shock conditions is associated with a non-linear increase in meiotic recombination frequency. We also find an independent effect of heat shock on organismal fitness, with fitness decreasing with increased duration of thermal stress.
Conclusions: Our results thus support the foundation of the FAR model for the evolution of plastic recombination. Our data also suggest that modulating recombination frequency is one mechanism by which organisms can rapidly respond to environmental cues and confer increased adaptive potential to their offspring.
Figures
Similar articles
-
Experimental evolution across different thermal regimes yields genetic divergence in recombination fraction but no divergence in temperature associated plastic recombination.Evolution. 2018 Apr;72(4):989-999. doi: 10.1111/evo.13454. Epub 2018 Mar 25. Evolution. 2018. PMID: 29468654
-
The effects of recombination-defective meiotic mutants in Drosophila melanogaster on gonial recombination in males.Mutat Res. 1979 Jul;61(2):221-7. doi: 10.1016/0027-5107(79)90129-5. Mutat Res. 1979. PMID: 113674
-
Genetic Background, Maternal Age, and Interaction Effects Mediate Rates of Crossing Over in Drosophila melanogaster Females.G3 (Bethesda). 2016 May 3;6(5):1409-16. doi: 10.1534/g3.116.027631. G3 (Bethesda). 2016. PMID: 26994290 Free PMC article.
-
[Genetic control of meiosis in Drozophila].Genetika. 2000 Oct;36(10):1301-21. Genetika. 2000. PMID: 11094742 Review. Russian.
-
Recombination rate plasticity: revealing mechanisms by design.Philos Trans R Soc Lond B Biol Sci. 2017 Dec 19;372(1736):20160459. doi: 10.1098/rstb.2016.0459. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 29109222 Free PMC article. Review.
Cited by
-
Desiccation-induced changes in recombination rate and crossover interference in Drosophila melanogaster: evidence for fitness-dependent plasticity.Genetica. 2019 Aug;147(3-4):291-302. doi: 10.1007/s10709-019-00070-6. Epub 2019 Jun 25. Genetica. 2019. PMID: 31240599
-
Linked-read sequencing of gametes allows efficient genome-wide analysis of meiotic recombination.Nat Commun. 2019 Sep 20;10(1):4310. doi: 10.1038/s41467-019-12209-2. Nat Commun. 2019. PMID: 31541084 Free PMC article.
-
Variation in Recombination Rate Is Shaped by Domestication and Environmental Conditions in Barley.Mol Biol Evol. 2019 Sep 1;36(9):2029-2039. doi: 10.1093/molbev/msz141. Mol Biol Evol. 2019. PMID: 31209472 Free PMC article.
-
Leagues of their own: sexually dimorphic features of meiotic prophase I.Chromosoma. 2019 Sep;128(3):199-214. doi: 10.1007/s00412-019-00692-x. Epub 2019 Mar 2. Chromosoma. 2019. PMID: 30826870 Free PMC article.
-
Seasonal changes in recombination characteristics in a natural population of Drosophila melanogaster.Heredity (Edinb). 2021 Sep;127(3):278-287. doi: 10.1038/s41437-021-00449-2. Epub 2021 Jun 23. Heredity (Edinb). 2021. PMID: 34163036 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

