Optical control of NMDA receptors with a diffusible photoswitch
- PMID: 26311290
- PMCID: PMC4560805
- DOI: 10.1038/ncomms9076
Optical control of NMDA receptors with a diffusible photoswitch
Abstract
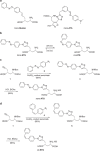
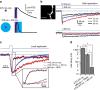
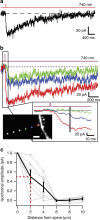
N-methyl-D-aspartate receptors (NMDARs) play a central role in synaptic plasticity, learning and memory, and are implicated in various neuronal disorders. We synthesized a diffusible photochromic glutamate analogue, azobenzene-triazole-glutamate (ATG), which is specific for NMDARs and functions as a photoswitchable agonist. ATG is inactive in its dark-adapted trans-isoform, but can be converted into its active cis-isoform using one-photon (near UV) or two-photon (740 nm) excitation. Irradiation with violet light photo-inactivates ATG within milliseconds, allowing agonist removal on the timescale of NMDAR deactivation. ATG is compatible with Ca(2+) imaging and can be used to optically mimic synaptic coincidence detection protocols. Thus, ATG can be used like traditional caged glutamate compounds, but with the added advantages of NMDAR specificity, low antagonism of GABAR-mediated currents, and precise temporal control of agonist delivery.
Figures




References
-
- Paoletti P., Bellone C. & Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013) . - PubMed
-
- Lester R. A., Clements J. D., Westbrook G. L. & Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature 346, 565–567 (1990) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

