MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics
- PMID: 26311337
- PMCID: PMC4722234
- DOI: 10.1038/ijo.2015.170
MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics
Abstract
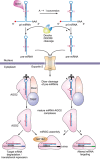
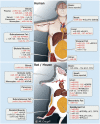
The prevalence of overweight and obesity in developed and developing countries has greatly increased the risk of insulin resistance and type 2 diabetes mellitus. It is evident from human and animal studies that obesity alters microRNA (miRNA) expression in metabolically important organs, and that miRNAs are involved in changes to normal physiology, acting as mediators of disease. miRNAs regulate multiple pathways including insulin signaling, immune-mediated inflammation, adipokine expression, adipogenesis, lipid metabolism, and food intake regulation. Thus, miRNA-based therapeutics represent an innovative and attractive treatment modality, with non-human primate studies showing great promise. In addition, miRNA measures in plasma or bodily fluids may be used as disease biomarkers and predictors of metabolic disease in humans. This review analyzes the role of miRNAs in obesity and insulin resistance, focusing on the miR-17/92, miR-143-145, miR-130, let-7, miR-221/222, miR-200, miR-223, miR-29 and miR-375 families, as well as miRNA changes by relevant tissue (adipose, liver and skeletal muscle). Further, the current and future applications of miRNA-based therapeutics and diagnostics in metabolic disease are discussed.
Figures
References
-
- 2Fullston T, Ohlsson Teague EM, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 2013; 27: 4226–4243. - PubMed
-
- 3Gonzalez-Bulnes A, Astiz S, Ovilo C, Lopez-Bote CJ, Sanchez-Sanchez R, Perez-Solana ML et al. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J Endocrinol 2014; 223: M17–M29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

