Digoxin inhibits PDGF-BB-induced VSMC proliferation and migration through an increase in ILK signaling and attenuates neointima formation following carotid injury
- PMID: 26311435
- PMCID: PMC4564091
- DOI: 10.3892/ijmm.2015.2320
Digoxin inhibits PDGF-BB-induced VSMC proliferation and migration through an increase in ILK signaling and attenuates neointima formation following carotid injury
Abstract
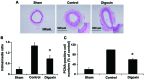
The increased proliferation and migration of vascular smooth muscle cells (VSMCs) are key events in the development of artery restenosis following percutaneous coronary intervention. Digoxin has long been used in the treatment of heart failure and has been shown to inhibit the proliferation of cancer cells through multiple pathways. However, the potential role of digoxin in the regulation of VSMC proliferation and migration and its effectiveness in the treatment of cardiovascular diseases, such as restenosis, remains unexplored. In the present study, we demonstrate that digoxin-induced growth inhibition is associated with the downregulation of CDK activation and the restoration of p27Kip1 levels in platelet-derived growth factor (PDGF)-stimulated VSMCs. In addition, we found that digoxin restored the PDGF‑BB-induced inhibition of integrin linked kinase (ILK) expression and prevented the PDGF‑BB-induced activation of glycogen synthase kinase (GSK)-3β. Furthermore, digoxin inhibited adhesion molecule and extracellular matrix relative protein expression. Finally, we found that digoxin significantly inhibited neointima formation, accompanied by a decrease in cell proliferation following vascular injury in rats. These effects of digoxin were shown to be mediated, at least in part, through an increase in ILK/Akt signaling and a decrease in GSK-3β signaling in PDGF‑BB-stimulated VSMCs. In conclusion, our data demonstrate that digoxin exerts an inhibitory effect on the PDGF‑BB-induced proliferation, migration and phenotypic modulation of VSMCs, and prevents neointima formation in rats. These observations indicate the potential therapeutic application of digoxin in the treatment of cardiovascular diseases, such as restenosis.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

