PAQR3 modulates cholesterol homeostasis by anchoring Scap/SREBP complex to the Golgi apparatus
- PMID: 26311497
- PMCID: PMC4560833
- DOI: 10.1038/ncomms9100
PAQR3 modulates cholesterol homeostasis by anchoring Scap/SREBP complex to the Golgi apparatus
Abstract
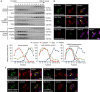
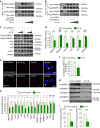
Cholesterol biosynthesis is regulated by transcription factors SREBPs and their escort protein Scap. On sterol depletion, Scap/SREBP complex is transported from endoplasmic reticulum (ER) to the Golgi apparatus where SREBP is activated. Under cholesterol sufficient condition, Insigs act as anchor proteins to retain Scap/SREBP in the ER. However, the anchor protein of Scap/SREBP in the Golgi is unknown. Here we report that a Golgi-localized membrane protein progestin and adipoQ receptors 3 (PAQR3) interacts with Scap and SREBP and tethers them to the Golgi. PAQR3 promotes Scap/SREBP complex formation, potentiates SREBP processing and enhances lipid synthesis. The mutually exclusive interaction between Scap and PAQR3 or Insig-1 is regulated by cholesterol level. PAQR3 knockdown in liver blunts SREBP pathway and decreases hepatic cholesterol content. Disrupting the interaction of PAQR3 with Scap/SREBP by a synthetic peptide inhibits SREBP processing and activation. Thus, PAQR3 regulates cholesterol homeostasis by anchoring Scap/SREBP to the Golgi and disruption of such function reduces cholesterol biosynthesis.
Figures





Similar articles
-
Cideb controls sterol-regulated ER export of SREBP/SCAP by promoting cargo loading at ER exit sites.EMBO J. 2019 Apr 15;38(8):e100156. doi: 10.15252/embj.2018100156. Epub 2019 Mar 11. EMBO J. 2019. PMID: 30858281 Free PMC article.
-
Sterol regulatory element-binding protein (SREBP) cleavage regulates Golgi-to-endoplasmic reticulum recycling of SREBP cleavage-activating protein (SCAP).J Biol Chem. 2014 Mar 14;289(11):7547-57. doi: 10.1074/jbc.M113.545699. Epub 2014 Jan 29. J Biol Chem. 2014. PMID: 24478315 Free PMC article.
-
Fatostatin blocks ER exit of SCAP but inhibits cell growth in a SCAP-independent manner.J Lipid Res. 2016 Aug;57(8):1564-73. doi: 10.1194/jlr.M069583. Epub 2016 Jun 20. J Lipid Res. 2016. PMID: 27324795 Free PMC article.
-
Retrospective on Cholesterol Homeostasis: The Central Role of Scap.Annu Rev Biochem. 2018 Jun 20;87:783-807. doi: 10.1146/annurev-biochem-062917-011852. Epub 2017 Aug 25. Annu Rev Biochem. 2018. PMID: 28841344 Free PMC article. Review.
-
Dual functions of Insig proteins in cholesterol homeostasis.Lipids Health Dis. 2012 Dec 18;11:173. doi: 10.1186/1476-511X-11-173. Lipids Health Dis. 2012. PMID: 23249523 Free PMC article. Review.
Cited by
-
Progestin and AdipoQ Receptor 3 Upregulates Fibronectin and Intercellular Adhesion Molecule-1 in Glomerular Mesangial Cells via Activating NF-κB Signaling Pathway Under High Glucose Conditions.Front Endocrinol (Lausanne). 2018 Jun 7;9:275. doi: 10.3389/fendo.2018.00275. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29930535 Free PMC article.
-
Nucleus-exported CLOCK acetylates PRPS to promote de novo nucleotide synthesis and liver tumour growth.Nat Cell Biol. 2023 Feb;25(2):273-284. doi: 10.1038/s41556-022-01061-0. Epub 2023 Jan 16. Nat Cell Biol. 2023. PMID: 36646788
-
FBXW7β loss-of-function enhances FASN-mediated lipogenesis and promotes colorectal cancer growth.Signal Transduct Target Ther. 2023 May 19;8(1):187. doi: 10.1038/s41392-023-01405-8. Signal Transduct Target Ther. 2023. PMID: 37202390 Free PMC article.
-
The role and mechanism of tetramethylpyrazine for atherosclerosis in animal models: A systematic review and meta-analysis.PLoS One. 2022 May 2;17(5):e0267968. doi: 10.1371/journal.pone.0267968. eCollection 2022. PLoS One. 2022. PMID: 35500001 Free PMC article.
-
The interaction between polyphyllin I and SQLE protein induces hepatotoxicity through SREBP-2/HMGCR/SQLE/LSS pathway.J Pharm Anal. 2023 Jan;13(1):39-54. doi: 10.1016/j.jpha.2022.11.005. Epub 2022 Nov 19. J Pharm Anal. 2023. PMID: 36820075 Free PMC article.
References
-
- Bloch K. The biological synthesis of cholesterol. Science 150, 19–28 (1965) . - PubMed
-
- Bucher N. L., Mc G. K., Gould E. & Loud A. V. Cholesterol biosynthesis in preparations of liver from normal, fasting, x-irradiated, cholesterol-fed, triton, or delta 4-cholesten-3-one-treated rats. J. Biol. Chem. 234, 262–267 (1959) . - PubMed
-
- Goldstein J. L., DeBose-Boyd R. A. & Brown M. S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

