A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo
- PMID: 26311731
- PMCID: PMC4934375
- DOI: 10.1126/scitranslmed.aac9459
A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo
Abstract
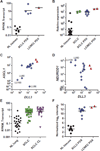
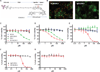
The high-grade pulmonary neuroendocrine tumors, small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC), remain among the most deadly malignancies. Therapies that effectively target and kill tumor-initiating cells (TICs) in these cancers should translate to improved patient survival. Patient-derived xenograft (PDX) tumors serve as excellent models to study tumor biology and characterize TICs. Increased expression of delta-like 3 (DLL3) was discovered in SCLC and LCNEC PDX tumors and confirmed in primary SCLC and LCNEC tumors. DLL3 protein is expressed on the surface of tumor cells but not in normal adult tissues. A DLL3-targeted antibody-drug conjugate (ADC), SC16LD6.5, comprised of a humanized anti-DLL3 monoclonal antibody conjugated to a DNA-damaging pyrrolobenzodiazepine (PBD) dimer toxin, induced durable tumor regression in vivo across multiple PDX models. Serial transplantation experiments executed with limiting dilutions of cells provided functional evidence confirming that the lack of tumor recurrence after SC16LD6.5 exposure resulted from effective targeting of DLL3-expressing TICs. In vivo efficacy correlated with DLL3 expression, and responses were observed in PDX models initiated from patients with both limited and extensive-stage disease and were independent of their sensitivity to standard-of-care chemotherapy regimens. SC16LD6.5 effectively targets and eradicates DLL3-expressing TICs in SCLC and LCNEC PDX tumors and is a promising first-in-class ADC for the treatment of high-grade pulmonary neuroendocrine tumors.
Copyright © 2015, American Association for the Advancement of Science.
Figures



Comment in
-
Targeting the seeds of small cell lung cancer.Ann Transl Med. 2017 Mar;5(5):113. doi: 10.21037/atm.2017.01.74. Ann Transl Med. 2017. PMID: 28361078 Free PMC article.
References
-
- Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch. Pathol. Lab. Med. 2010;134:1628–1638. - PubMed
-
- Travis WD. Pathology and diagnosis of neuroendocrine tumors: Lung neuroendocrine. Thorac. Surg. Clin. 2014;24:257–266. - PubMed
-
- William WN, Jr, Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat. Rev. Clin. Oncol. 2011;8:611–619. - PubMed
-
- Joshi M, Ayoola A, Belani CP. Small-cell lung cancer: An update on targeted therapies. Adv. Exp. Med. Biol. 2013;779:385–404. - PubMed
-
- Eichhorn F, Dienemann H, Muley T, Warth A, Hoffmann H. Predictors of survival after operation among patients with large cell neuroendocrine carcinoma of the lung. Ann. Thorac. Surg. 2015;99:983–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

