Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study
- PMID: 26311750
- PMCID: PMC4603595
- DOI: 10.1212/WNL.0000000000001950
Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study
Abstract
Objective: We aimed to perform an observational study of age at loss of independent ambulation (LoA) and side-effect profiles associated with different glucocorticoid corticosteroid (GC) regimens in Duchenne muscular dystrophy (DMD).
Methods: We studied 340 participants in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG-DNHS). LoA was defined as continuous wheelchair use. Effects of prednisone or prednisolone (PRED)/deflazacort (DFZ), administration frequency, and dose were analyzed by time-varying Cox regression. Side-effect frequencies were compared using χ(2) test.
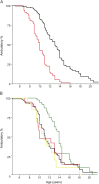
Results: Participants treated ≥1 year while ambulatory (n = 252/340) showed a 3-year median delay in LoA (p < 0.001). Fourteen different regimens were observed. Nondaily treatment was common for PRED (37%) and rare for DFZ (3%). DFZ was associated with later LoA than PRED (hazard ratio 0.294 ± 0.053 vs 0.490 ± 0.08, p = 0.003; 2-year difference in median LoA with daily administration, p < 0.001). Average dose was lower for daily PRED (0.56 mg/kg/d, 75% of recommended) than daily DFZ (0.75 mg/kg/d, 83% of recommended, p < 0.001). DFZ showed higher frequencies of growth delay (p < 0.001), cushingoid appearance (p = 0.002), and cataracts (p < 0.001), but not weight gain.
Conclusions: Use of DFZ was associated with later LoA and increased frequency of side effects. Differences in standards of care and dosing complicate interpretation of this finding, but stratification by PRED/DFZ might be considered in clinical trials. This study emphasizes the necessity of a randomized, blinded trial of GC regimens in DMD.
Classification of evidence: This study provides Class IV evidence that GCs are effective in delaying LoA in patients with DMD.
© 2015 American Academy of Neurology.
Figures
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928. - PubMed
-
- Moxley RT, III, Ashwal S, Pandya S, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005;64:13–20. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy: part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77–93. - PubMed
-
- Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med 1989;320:1592–1597. - PubMed
-
- Griggs RC, Moxley RT, III, Mendell JR, et al. Prednisone in Duchenne dystrophy: a randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol 1991;48:383–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
