Bexarotene-Activated Retinoid X Receptors Regulate Neuronal Differentiation and Dendritic Complexity
- PMID: 26311769
- PMCID: PMC4549399
- DOI: 10.1523/JNEUROSCI.1001-15.2015
Bexarotene-Activated Retinoid X Receptors Regulate Neuronal Differentiation and Dendritic Complexity
Abstract
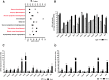
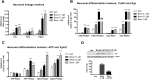
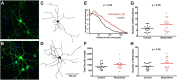
Bexarotene-activated retinoid X receptors (RXRs) ameliorate memory deficits in Alzheimer's disease mouse models, including mice expressing human apolipoprotein E (APOE) isoforms. The goal of this study was to gain further insight into molecular mechanisms whereby ligand-activated RXR can affect or restore cognitive functions. We used an unbiased approach to discover genome-wide changes in RXR cistrome (ChIP-Seq) and gene expression profile (RNA-Seq) in response to bexarotene in the cortex of APOE4 mice. Functional categories enriched in both datasets revealed that bexarotene-liganded RXR affected signaling pathways associated with neurogenesis and neuron projection development. To further validate the significance of RXR for these functions, we used mouse embryonic stem (ES) cells, primary neurons, and APOE3 and APOE4 mice treated with bexarotene. In vitro data from ES cells confirmed that bexarotene-activated RXR affected neuronal development at different levels, including proliferation of neural progenitors and neuronal differentiation, and stimulated neurite outgrowth. This effect was validated in vivo by demonstrating an increased number of neuronal progenitors after bexarotene treatment in the dentate gyrus of APOE3 and APOE4 mice. In primary neurons, bexarotene enhanced the dendritic complexity characterized by increased branching, intersections, and bifurcations. This effect was confirmed by in vivo studies demonstrating that bexarotene significantly improved the compromised dendritic structure in the hippocampus of APOE4 mice. We conclude that bexarotene-activated RXRs promote genetic programs involved in the neurogenesis and development of neuronal projections and these results have significance for the improvement of cognitive deficits.
Significance statement: Bexarotene-activated retinoid X receptors (RXRs) ameliorate memory deficits in Alzheimer's disease mouse models, including mice expressing human apolipoprotein E (APOE) isoforms. The goal of this study was to gain further insight into molecular mechanisms whereby ligand-activated RXR can affect or restore cognitive functions. We used an unbiased approach to discover genome-wide changes in RXR cistrome (ChIP-Seq) and gene expression profile (RNA-Seq) in response to bexarotene in the cortex of APOE4 mice. Functional categories enriched in both datasets revealed that liganded RXR affected signaling pathways associated with neurogenesis and neuron projection development. The significance of RXR for these functions was validated in mouse embryonic stem cells, primary neurons, and APOE3 and APOE4 mice treated with bexarotene.
Keywords: APOE4 and APOE3; ChIP-Seq/RNA-Seq; adult neurogenesis; bexarotene; neuronal differentiation; retinoid X receptor.
Copyright © 2015 the authors 0270-6474/15/3511862-15$15.00/0.
Figures






References
-
- Akama K, Horikoshi T, Nakayama T, Otsu M, Imaizumi N, Nakamura M, Toda T, Inuma M, Hirano H, Kondo Y, Suzuki Y, Inoue N. Proteomic identification of differentially expressed genes during differentiation of cynomolgus monkey (Macaca fascicularis) embryonic stem cells to astrocyte progenitor cells in vitro. Biochim Biophys Acta. 2013;1834:601–610. doi: 10.1016/j.bbapap.2012.12.002. - DOI - PubMed
-
- Artavanis-Tsakonas S, Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991;7:403–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
