Spatiotemporal Profile of Voltage-Sensitive Dye Responses in the Visual Cortex of Tree Shrews Evoked by Electric Microstimulation of the Dorsal Lateral Geniculate and Pulvinar Nuclei
- PMID: 26311771
- PMCID: PMC4549400
- DOI: 10.1523/JNEUROSCI.0717-15.2015
Spatiotemporal Profile of Voltage-Sensitive Dye Responses in the Visual Cortex of Tree Shrews Evoked by Electric Microstimulation of the Dorsal Lateral Geniculate and Pulvinar Nuclei
Abstract
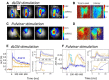
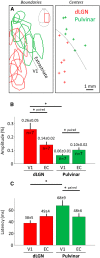
The primary visual cortex (V1) receives its main thalamic drive from the dorsal lateral geniculate nucleus (dLGN) through synaptic contacts terminating primarily in cortical layer IV. In contrast, the projections from the pulvinar nucleus to the cortex are less clearly defined. The pulvinar projects predominantly to layer I in V1, and layer IV in extrastriate areas. These projection patterns suggest that the pulvinar nucleus most strongly influences (drives) activity in cortical areas beyond V1. Should this hypothesis be true, one would expect the spatiotemporal responses evoked by pulvinar activation to be different in V1 and extrastriate areas, reflecting the different connectivity patterns. We investigated this issue by analyzing the spatiotemporal dynamics of cortical visual areas' activity following thalamic electrical microstimulation in tree shrews, using optical imaging and voltage-sensitive dyes. As expected, electrical stimulation of the dLGN induced fast and local responses in V1, as well as in extrastriate and contralateral cortical areas. In contrast, electrical stimulation of the pulvinar induced fast and local responses in extrastriate areas, followed by weak and diffuse activation in V1 and contralateral cortical areas. This study highlights spatiotemporal cortical activation characteristics induced by stimulation of first (dLGN) and high-order (pulvinar) thalamic nuclei.
Significance statement: The pulvinar nucleus represents the main extrageniculate thalamic visual structure in higher-order mammals, but its exact role remains enigmatic. The pulvinar receive prominent inputs from virtually all visual cortical areas. Cortico-thalamo-cortical pathways through the pulvinar nuclei may then provide a complementary route for corticocortical information flow. One step toward the understanding of the role of transthalamic corticocortical pathways is to determine the nature of the signals transmitted between the cortex and the thalamus. By performing, for the first time, high spatiotemporal mesoscopic imaging on tree shrews (the primate's closest relative) through the combination of voltage-sensitive dye recordings and brain stimulation, we revealed clear evidence of distinct thalamocortical functional connectivity pattern originating from the geniculate nucleus and the pulvinar nuclei.
Keywords: driver; geniculate nucleus; modulator; pulvinar; thalamocortical connections.
Copyright © 2015 the authors 0270-6474/15/3511891-06$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
