Trans-Modulation of the Somatostatin Type 2A Receptor Trafficking by Insulin-Regulated Aminopeptidase Decreases Limbic Seizures
- PMID: 26311777
- PMCID: PMC6705451
- DOI: 10.1523/JNEUROSCI.0476-15.2015
Trans-Modulation of the Somatostatin Type 2A Receptor Trafficking by Insulin-Regulated Aminopeptidase Decreases Limbic Seizures
Abstract
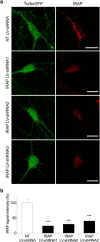
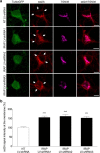
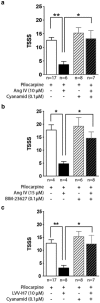
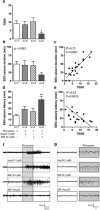
Within the hippocampus, the major somatostatin (SRIF) receptor subtype, the sst2A receptor, is localized at postsynaptic sites of the principal neurons where it modulates neuronal activity. Following agonist exposure, this receptor rapidly internalizes and recycles slowly through the trans-Golgi network. In epilepsy, a high and chronic release of somatostatin occurs, which provokes, in both rat and human tissue, a decrease in the density of this inhibitory receptor at the cell surface. The insulin-regulated aminopeptidase (IRAP) is involved in vesicular trafficking and shares common regional distribution with the sst2A receptor. In addition, IRAP ligands display anticonvulsive properties. We therefore sought to assess by in vitro and in vivo experiments in hippocampal rat tissue whether IRAP ligands could regulate the trafficking of the sst2A receptor and, consequently, modulate limbic seizures. Using pharmacological and cell biological approaches, we demonstrate that IRAP ligands accelerate the recycling of the sst2A receptor that has internalized in neurons in vitro or in vivo. Most importantly, because IRAP ligands increase the density of this inhibitory receptor at the plasma membrane, they also potentiate the neuropeptide SRIF inhibitory effects on seizure activity. Our results further demonstrate that IRAP is a therapeutic target for the treatment of limbic seizures and possibly for other neurological conditions in which downregulation of G-protein-coupled receptors occurs.
Significance statement: The somatostatin type 2A receptor (sst2A) is localized on principal hippocampal neurons and displays anticonvulsant properties. Following agonist exposure, however, this receptor rapidly internalizes and recycles slowly. The insulin-regulated aminopeptidase (IRAP) is involved in vesicular trafficking and shares common regional distribution with the sst2A receptor. We therefore assessed by in vitro and in vivo experiments whether IRAP could regulate the trafficking of this receptor. We demonstrate that IRAP ligands accelerate sst2A recycling in hippocampal neurons. Because IRAP ligands increase the density of sst2A receptors at the plasma membrane, they also potentiate the effects of this inhibitory receptor on seizure activity. Our results further demonstrate that IRAP is a therapeutic target for the treatment of limbic seizures.
Keywords: GPCR; insulin-regulated aminopeptidase; limbic seizures; neuropeptide; somatostatin; traffic.
Copyright © 2015 the authors 0270-6474/15/3511961-16$15.00/0.
Figures






Similar articles
-
Ligand-dependent mechanisms of sst2A receptor trafficking: role of site-specific phosphorylation and receptor activation in the actions of biased somatostatin agonists.Mol Endocrinol. 2011 Jun;25(6):1040-54. doi: 10.1210/me.2010-0398. Epub 2011 Apr 14. Mol Endocrinol. 2011. PMID: 21493671 Free PMC article.
-
Sub-cellular localization of insulin-regulated membrane aminopeptidase, IRAP to vesicles in neurons.J Neurochem. 2007 Aug;102(3):967-76. doi: 10.1111/j.1471-4159.2007.04659.x. Epub 2007 May 14. J Neurochem. 2007. PMID: 17504262
-
Involvement of the somatostatin-2 receptor in the anti-convulsant effect of angiotensin IV against pilocarpine-induced limbic seizures in rats.J Neurochem. 2006 Aug;98(4):1100-13. doi: 10.1111/j.1471-4159.2006.03942.x. Epub 2006 Jun 12. J Neurochem. 2006. PMID: 16771832
-
On the role of somatostatin in seizure control: clues from the hippocampus.Rev Neurosci. 2003;14(3):285-301. doi: 10.1515/revneuro.2003.14.3.285. Rev Neurosci. 2003. PMID: 14513869 Review.
-
Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis.Eur J Neurosci. 1999 Nov;11(11):3767-76. doi: 10.1046/j.1460-9568.1999.00838.x. Eur J Neurosci. 1999. PMID: 10583466 Review.
Cited by
-
From Angiotensin IV to Small Peptidemimetics Inhibiting Insulin-Regulated Aminopeptidase.Front Pharmacol. 2020 Oct 15;11:590855. doi: 10.3389/fphar.2020.590855. eCollection 2020. Front Pharmacol. 2020. PMID: 33178027 Free PMC article. Review.
-
Is There an Interplay Between the Functional Domains of IRAP?Front Cell Dev Biol. 2020 Sep 29;8:585237. doi: 10.3389/fcell.2020.585237. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33134302 Free PMC article. Review.
-
Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes.Basic Res Cardiol. 2021 Oct 28;116(1):63. doi: 10.1007/s00395-021-00895-3. Basic Res Cardiol. 2021. PMID: 34713358 Free PMC article.
-
Reciprocal Expression Patterns of Placental Leucine Aminopeptidase/Insulin-Regulated Aminopeptidase and Vasopressin in the Murine Brain.Front Mol Biosci. 2020 Jul 24;7:168. doi: 10.3389/fmolb.2020.00168. eCollection 2020. Front Mol Biosci. 2020. PMID: 32793633 Free PMC article.
-
A simple novel approach for detecting blood-brain barrier permeability using GPCR internalization.Neuropathol Appl Neurobiol. 2021 Feb;47(2):297-315. doi: 10.1111/nan.12665. Epub 2020 Sep 27. Neuropathol Appl Neurobiol. 2021. PMID: 32898926 Free PMC article.
References
-
- Albiston AL, Diwakarla S, Fernando RN, Mountford SJ, Yeatman HR, Morgan B, Pham V, Holien JK, Parker MW, Thompson PE, Chai SY. Identification and development of specific inhibitors for insulin-regulated aminopeptidase as a new class of cognitive enhancers. Br J Pharmacol. 2011;164:37–47. doi: 10.1111/j.1476-5381.2011.01402.x. - DOI - PMC - PubMed
-
- Aourz N, De Bundel D, Stragier B, Clinckers R, Portelli J, Michotte Y, Smolders I. Rat hippocampal somatostatin sst3 and sst4 receptors mediate anticonvulsive effects in vivo: indications of functional interactions with sst2 receptors. Neuropharmacology. 2011;61:1327–1333. doi: 10.1016/j.neuropharm.2011.08.003. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
