Defects of Lipid Synthesis Are Linked to the Age-Dependent Demyelination Caused by Lamin B1 Overexpression
- PMID: 26311780
- PMCID: PMC4549407
- DOI: 10.1523/JNEUROSCI.1668-15.2015
Defects of Lipid Synthesis Are Linked to the Age-Dependent Demyelination Caused by Lamin B1 Overexpression
Abstract
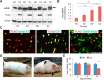
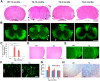
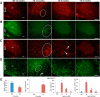
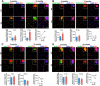
Lamin B1 is a component of the nuclear lamina and plays a critical role in maintaining nuclear architecture, regulating gene expression and modulating chromatin positioning. We have previously shown that LMNB1 gene duplications cause autosomal dominant leukodystrophy (ADLD), a fatal adult onset demyelinating disease. The mechanisms by which increased LMNB1 levels cause ADLD are unclear. To address this, we used a transgenic mouse model where Lamin B1 overexpression is targeted to oligodendrocytes. These mice showed severe vacuolar degeneration of the spinal cord white matter together with marked astrogliosis, microglial infiltration, and secondary axonal damage. Oligodendrocytes in the transgenic mice revealed alterations in histone modifications favoring a transcriptionally repressed state. Chromatin changes were accompanied by reduced expression of genes involved in lipid synthesis pathways, many of which are known to play important roles in myelin regulation and are preferentially expressed in oligodendrocytes. Decreased lipogenic gene expression resulted in a significant reduction in multiple classes of lipids involved in myelin formation. Many of these gene expression changes and lipid alterations were observed even before the onset of the phenotype, suggesting a causal role. Our findings establish, for the first time, a link between LMNB1 and lipid synthesis in oligodendrocytes, and provide a mechanistic framework to explain the age dependence and white matter involvement of the disease phenotype. These results have implications for disease pathogenesis and may also shed light on the regulation of lipid synthesis pathways in myelin maintenance and turnover.
Significance statement: Autosomal dominant leukodystrophy (ADLD) is fatal neurological disorder caused by increased levels of the nuclear protein, Lamin B1. The disease is characterized by an age-dependent loss of myelin, the fatty sheath that covers nerve fibers. We have studied a mouse model where Lamin B1 level are increased in oligodendrocytes, the cell type that produces myelin in the CNS. We demonstrate that destruction of myelin in the spinal cord is responsible for the degenerative phenotype in our mouse model. We show that this degeneration is mediated by reduced expression of lipid synthesis genes and the subsequent reduction in myelin enriched lipids. These findings provide a mechanistic framework to explain the age dependence and tissue specificity of the ADLD disease phenotype.
Keywords: Lamin B1; chromatin; demyelination; gene expression; inflammation; lipid.
Copyright © 2015 the authors 0270-6474/15/3512003-16$15.00/0.
Figures





References
-
- Bartoletti-Stella A, Gasparini L, Giacomini C, Corrado P, Terlizzi R, Giorgio E, Magini P, Seri M, Baruzzi A, Parchi P, Brusco A, Cortelli P, Capellari S. Messenger RNA processing is altered in autosomal dominant leukodystrophydagger. Hum Mol Genet. 2015;24:2746–2756. doi: 10.1093/hmg/ddv034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
