IGF-1R Reduction Triggers Neuroprotective Signaling Pathways in Spinal Muscular Atrophy Mice
- PMID: 26311784
- PMCID: PMC6705454
- DOI: 10.1523/JNEUROSCI.0608-15.2015
IGF-1R Reduction Triggers Neuroprotective Signaling Pathways in Spinal Muscular Atrophy Mice
Abstract
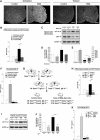
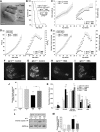
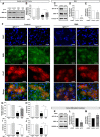
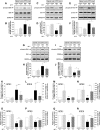
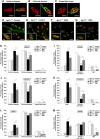
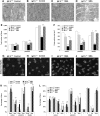
Spinal muscular atrophy (SMA) is a neuromuscular disease characterized by the selective loss of spinal motor neurons due to the depletion of the survival of motor neuron (SMN) protein. No therapy is currently available for SMA, which represents the leading genetic cause of death in childhood. In the present study, we report that insulin-like growth factor-1 receptor (Igf-1r) gene expression is enhanced in the spinal cords of SMA-like mice. The reduction of expression, either at the physiological (through physical exercise) or genetic level, resulted in the following: (1) a significant improvement in lifespan and motor behavior, (2) a significant motor neuron protection, and (3) an increase in SMN expression in spinal cord and skeletal muscles through both transcriptional and posttranscriptional mechanisms. Furthermore, we have found that reducing IGF-1R expression is sufficient to restore intracellular signaling pathway activation profile lying downstream of IGF-1R, resulting in both the powerful activation of the neuroprotective AKT/CREB pathway and the inhibition of the ERK and JAK pathways. Therefore, reducing rather than enhancing the IGF-1 pathway could constitute a useful strategy to limit neurodegeneration in SMA.
Significance statement: Recent evidence of IGF-1 axis alteration in spinal muscular atrophy (SMA), a very severe neurodegenerative disease affecting specifically the motor neurons, have triggered a renewed interest in insulin-like growth factor-1 (IGF-1) pathway activation as a potential therapeutic approach for motor neuron diseases. The present study challenges this point of view and brings the alternative hypothesis that reducing rather than enhancing the IGF-1 signaling pathway exerts a neuroprotective effect in SMA. Furthermore, the present data substantiate a newly emerging concept that the modulation of IGF-1 receptor expression is a key event selectively determining the activation level of intracellular pathways that lie downstream of the receptor. This aspect should be considered when designing IGF-1-based treatments for neurodegenerative diseases.
Keywords: IGF-1R; SMN; gene expression; model mice; signaling pathways; spinal muscular atrophy.
Copyright © 2015 the authors 0270-6474/15/3512063-17$15.00/0.
Figures


References
-
- Biondi O, Grondard C, Lécolle S, Deforges S, Pariset C, Lopes P, Cifuentes-Diaz C, Li H, della Gaspera B, Chanoine C, Charbonnier F. Exercise-induced activation of NMDA receptor promotes motor unit development and survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2008;28:953–962. doi: 10.1523/JNEUROSCI.3237-07.2008. - DOI - PMC - PubMed
-
- Biondi O, Branchu J, Sanchez G, Lancelin C, Deforges S, Lopes P, Pariset C, Lécolle S, Côté J, Chanoine C, Charbonnier F. In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice. J Neurosci. 2010;30:11288–11299. doi: 10.1523/JNEUROSCI.1764-10.2010. - DOI - PMC - PubMed
-
- Branchu J, Biondi O, Chali F, Collin T, Leroy F, Mamchaoui K, Makoukji J, Pariset C, Lopes P, Massaad C, Chanoine C, Charbonnier F. Shift from extracellular signal-regulated kinase to AKT/cAMP response element-binding protein pathway increases survival-motor-neuron expression in spinal-muscular-atrophy-like mice and patient cells. J Neurosci. 2013;33:4280–4294. doi: 10.1523/JNEUROSCI.2728-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
