Sinorhizobium meliloti Phage ΦM9 Defines a New Group of T4 Superfamily Phages with Unusual Genomic Features but a Common T=16 Capsid
- PMID: 26311868
- PMCID: PMC4621102
- DOI: 10.1128/JVI.01353-15
Sinorhizobium meliloti Phage ΦM9 Defines a New Group of T4 Superfamily Phages with Unusual Genomic Features but a Common T=16 Capsid
Abstract
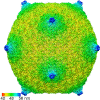
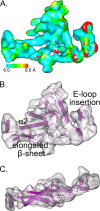
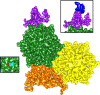
Relatively little is known about the phages that infect agriculturally important nitrogen-fixing rhizobial bacteria. Here we report the genome and cryo-electron microscopy structure of the Sinorhizobium meliloti-infecting T4 superfamily phage ΦM9. This phage and its close relative Rhizobium phage vB_RleM_P10VF define a new group of T4 superfamily phages. These phages are distinctly different from the recently characterized cyanophage-like S. meliloti phages of the ΦM12 group. Structurally, ΦM9 has a T=16 capsid formed from repeating units of an extended gp23-like subunit that assemble through interactions between one subunit and the adjacent E-loop insertion domain. Though genetically very distant from the cyanophages, the ΦM9 capsid closely resembles that of the T4 superfamily cyanophage Syn9. ΦM9 also has the same T=16 capsid architecture as the very distant phage SPO1 and the herpesviruses. Despite their overall lack of similarity at the genomic and structural levels, ΦM9 and S. meliloti phage ΦM12 have a small number of open reading frames in common that appear to encode structural proteins involved in interaction with the host and which may have been acquired by horizontal transfer. These proteins are predicted to encode tail baseplate proteins, tail fibers, tail fiber assembly proteins, and glycanases that cleave host exopolysaccharide.
Importance: Despite recent advances in the phylogenetic and structural characterization of bacteriophages, only a small number of phages of plant-symbiotic nitrogen-fixing soil bacteria have been studied at the molecular level. The effects of phage predation upon beneficial bacteria that promote plant growth remain poorly characterized. First steps in understanding these soil bacterium-phage dynamics are genetic, molecular, and structural characterizations of these groups of phages. The T4 superfamily phages are among the most complex phages; they have large genomes packaged within an icosahedral head and a long, contractile tail through which the DNA is delivered to host cells. This phylogenetic and structural study of S. meliloti-infecting T4 superfamily phage ΦM9 provides new insight into the diversity of this family. The comparison of structure-related genes in both ΦM9 and S. meliloti-infecting T4 superfamily phage ΦM12, which comes from a completely different lineage of these phages, allows the identification of host infection-related factors.
Copyright © 2015, Johnson et al.
Figures




References
-
- Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M. 2013. The global nitrogen cycle in the twenty-first century. Philos Trans R Soc Lond B Biol Sci. 368:20130164. doi:10.1098/rstb.2013.0164. - DOI - PMC - PubMed
-
- Sullivan MB, Huang KH, Ignacio-Espinoza JC, Berlin AM, Kelly L, Weigele PR, DeFrancesco AS, Kern SE, Thompson LR, Young S, Yandava C, Fu R, Krastins B, Chase M, Sarracino D, Osburne MS, Henn MR, Chisholm SW. 2010. Genomic analysis of oceanic cyanobacterial myoviruses compared with T4-like myoviruses from diverse hosts and environments. Environ Microbiol 12:3035–3056. doi:10.1111/j.1462-2920.2010.02280.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

