Remote Activation of Host Cell DNA Synthesis in Uninfected Cells Signaled by Infected Cells in Advance of Virus Transmission
- PMID: 26311877
- PMCID: PMC4621119
- DOI: 10.1128/JVI.01950-15
Remote Activation of Host Cell DNA Synthesis in Uninfected Cells Signaled by Infected Cells in Advance of Virus Transmission
Abstract
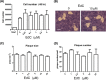
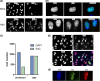
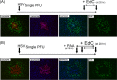
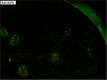
Viruses modulate cellular processes and metabolism in diverse ways, but these are almost universally studied in the infected cell itself. Here, we study spatial organization of DNA synthesis during multiround transmission of herpes simplex virus (HSV) using pulse-labeling with ethynyl nucleotides and cycloaddition of azide fluorophores. We report a hitherto unknown and unexpected outcome of virus-host interaction. Consistent with the current understanding of the single-step growth cycle, HSV suppresses host DNA synthesis and promotes viral DNA synthesis in spatially segregated compartments within the cell. In striking contrast, during progressive rounds of infection initiated at a single cell, we observe that infection induces a clear and pronounced stimulation of cellular DNA replication in remote uninfected cells. This induced DNA synthesis was observed in hundreds of uninfected cells at the extended border, outside the perimeter of the progressing infection. Moreover, using pulse-chase analysis, we show that this activation is maintained, resulting in a propagating wave of host DNA synthesis continually in advance of infection. As the virus reaches and infects these activated cells, host DNA synthesis is then shut off and replaced with virus DNA synthesis. Using nonpropagating viruses or conditioned medium, we demonstrate a paracrine effector of uninfected cell DNA synthesis in remote cells continually in advance of infection. These findings have significant implications, likely with broad applicability, for our understanding of the ways in which virus infection manipulates cell processes not only in the infected cell itself but also now in remote uninfected cells, as well as of mechanisms governing host DNA synthesis.
Importance: We show that during infection initiated by a single particle with progressive cell-cell virus transmission (i.e., the normal situation), HSV induces host DNA synthesis in uninfected cells, mediated by a virus-induced paracrine effector. The field has had no conception that this process occurs, and the work changes our interpretation of virus-host interaction during advancing infection and has implications for understanding controls of host DNA synthesis. Our findings demonstrate the utility of chemical biology techniques in analysis of infection processes, reveal distinct processes when infection is examined in multiround transmission versus single-step growth curves, and reveal a hitherto-unknown process in virus infection, likely relevant for other viruses (and other infectious agents) and for remote signaling of other processes, including transcription and protein synthesis.
Copyright © 2015, Schmidt et al.
Figures


Comment in
-
mSphere of Influence: Virology in the noise-how cell-to-cell variability impacts viral infection outcomes.mSphere. 2023 Oct 24;8(5):e0043823. doi: 10.1128/msphere.00438-23. Epub 2023 Sep 25. mSphere. 2023. PMID: 37747254 Free PMC article.
References
-
- Flint SJ, Enquist LW, Krug RM, Racaniello VR, Skalka AM. 2009. Principles of virology. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

