Bst2/Tetherin Is Induced in Neurons by Type I Interferon and Viral Infection but Is Dispensable for Protection against Neurotropic Viral Challenge
- PMID: 26311886
- PMCID: PMC4621123
- DOI: 10.1128/JVI.01745-15
Bst2/Tetherin Is Induced in Neurons by Type I Interferon and Viral Infection but Is Dispensable for Protection against Neurotropic Viral Challenge
Abstract
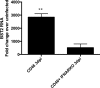
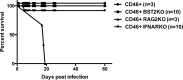
In permissive mouse central nervous system (CNS) neurons, measles virus (MV) spreads in the absence of hallmark viral budding or neuronal death, with transmission occurring efficiently and exclusively via the synapse. MV infection also initiates a robust type I interferon (IFN) response, resulting in the synthesis of a large number of genes, including bone marrow stromal antigen 2 (Bst2)/tetherin/CD317. Bst2 restricts the release of some enveloped viruses, but to date, its role in viral infection of neurons has not been assessed. Consequently, we investigated how Bst2 was induced and what role it played in MV neuronal infection. The magnitude of induction of neuronal Bst2 RNA and protein following IFN exposure and viral infection was notably higher than in similarly treated mouse embryo fibroblasts (MEFs). Bst2 synthesis was both IFN and Stat1 dependent. Although Bst2 prevented MV release from nonneuronal cells, its deletion had no effect on viral pathogenesis in MV-challenged mice. Our findings underscore how cell-type-specific differences impact viral infection and pathogenesis.
Importance: Viral infections of the central nervous system can lead to debilitating disease and death. Moreover, it is becoming increasingly clear that nonrenewable cells, including most central nervous system neurons, combat neurotropic viral infections in fundamentally different ways than other rapidly dividing and renewable cell populations. Here we identify type I interferon signaling as a key inducer of a known antiviral protein (Bst2) in neurons. Unexpectedly, the gene is dispensable for clearance of neurotropic viral infection despite its well-defined contribution to limiting the spread of enveloped viruses in proliferating cells. A deeper appreciation of the importance of cell type heterogeneity in antiviral immunity will aid in the identification of unique therapeutic targets for life-threatening viral infections.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Murine BST2/tetherin promotes measles virus infection of neurons.Virology. 2021 Nov;563:38-43. doi: 10.1016/j.virol.2021.08.005. Epub 2021 Aug 15. Virology. 2021. PMID: 34416448 Free PMC article.
-
STAT1-independent control of a neurotropic measles virus challenge in primary neurons and infected mice.J Immunol. 2012 Feb 15;188(4):1915-23. doi: 10.4049/jimmunol.1101356. Epub 2012 Jan 13. J Immunol. 2012. PMID: 22246627 Free PMC article.
-
Cutting edge: paradoxical roles of BST2/tetherin in promoting type I IFN response and viral infection.J Immunol. 2012 Mar 15;188(6):2488-92. doi: 10.4049/jimmunol.1103145. Epub 2012 Feb 10. J Immunol. 2012. PMID: 22327075 Free PMC article.
-
Making it to the synapse: measles virus spread in and among neurons.Curr Top Microbiol Immunol. 2009;330:3-30. doi: 10.1007/978-3-540-70617-5_1. Curr Top Microbiol Immunol. 2009. PMID: 19203102 Free PMC article. Review.
-
Multi-functional BST2/tetherin against HIV-1, other viruses and LINE-1.Front Cell Infect Microbiol. 2022 Sep 13;12:979091. doi: 10.3389/fcimb.2022.979091. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36176574 Free PMC article. Review.
Cited by
-
Analysis of tick-borne encephalitis virus-induced host responses in human cells of neuronal origin and interferon-mediated protection.J Gen Virol. 2017 Aug;98(8):2043-2060. doi: 10.1099/jgv.0.000853. Epub 2017 Aug 8. J Gen Virol. 2017. PMID: 28786780 Free PMC article.
-
Host Cell Restriction Factors Blocking Efficient Vector Transduction: Challenges in Lentiviral and Adeno-Associated Vector Based Gene Therapies.Cells. 2023 Feb 24;12(5):732. doi: 10.3390/cells12050732. Cells. 2023. PMID: 36899868 Free PMC article. Review.
-
BST2/Tetherin Overexpression Modulates Morbillivirus Glycoprotein Production to Inhibit Cell-Cell Fusion.Viruses. 2019 Jul 30;11(8):692. doi: 10.3390/v11080692. Viruses. 2019. PMID: 31366072 Free PMC article.
-
Keeping it in check: chronic viral infection and antiviral immunity in the brain.Nat Rev Neurosci. 2016 Dec;17(12):766-776. doi: 10.1038/nrn.2016.140. Epub 2016 Nov 4. Nat Rev Neurosci. 2016. PMID: 27811921 Free PMC article. Review.
-
Bone Marrow Stromal Antigen 2 is a Potential Unfavorable Prognostic Factor for High-Grade Glioma.Onco Targets Ther. 2020 Aug 26;13:8723-8734. doi: 10.2147/OTT.S258631. eCollection 2020. Onco Targets Ther. 2020. PMID: 32943880 Free PMC article.
References
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol 177:3260–3265. doi:10.4049/jimmunol.177.5.3260. - DOI - PubMed
-
- Goto T, Kennel SJ, Abe M, Takishita M, Kosaka M, Solomon A, Saito S. 1994. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood 84:1922–1930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

