Cross-Species Infectivity of H3N8 Influenza Virus in an Experimental Infection in Swine
- PMID: 26311894
- PMCID: PMC4645675
- DOI: 10.1128/JVI.01509-15
Cross-Species Infectivity of H3N8 Influenza Virus in an Experimental Infection in Swine
Abstract
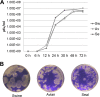
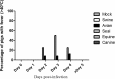
Avian influenza A viruses have gained increasing attention due to their ability to cross the species barrier and cause severe disease in humans and other mammal species as pigs. H3 and particularly H3N8 viruses, are highly adaptive since they are found in multiple avian and mammal hosts. H3N8 viruses have not been isolated yet from humans; however, a recent report showed that equine influenza A viruses (IAVs) can be isolated from pigs, although an established infection has not been observed thus far in this host. To gain insight into the possibility of H3N8 avian IAVs to cross the species barrier into pigs, in vitro experiments and an experimental infection in pigs with four H3N8 viruses from different origins (equine, canine, avian, and seal) were performed. As a positive control, an H3N2 swine influenza virus A was used. Although equine and canine viruses hardly replicated in the respiratory systems of pigs, avian and seal viruses replicated substantially and caused detectable lesions in inoculated pigs without previous adaptation. Interestingly, antibodies against hemagglutinin could not be detected after infection by hemagglutination inhibition (HAI) test with avian and seal viruses. This phenomenon was observed not only in pigs but also in mice immunized with the same virus strains. Our data indicated that H3N8 IAVs from wild aquatic birds have the potential to cross the species barrier and establish successful infections in pigs that might spread unnoticed using the HAI test as diagnostic tool.
Importance: Although natural infection of humans with an avian H3N8 influenza A virus has not yet been reported, this influenza A virus subtype has already crossed the species barrier. Therefore, we have examined the potential of H3N8 from canine, equine, avian, and seal origin to productively infect pigs. Our results demonstrated that avian and seal viruses replicated substantially and caused detectable lesions in inoculated pigs without previous adaptation. Surprisingly, we could not detect specific antibodies against hemagglutinin in any H3N8-infected pigs. Therefore, special attention should be focused toward viruses of the H3N8 subtype since they could behave as stealth viruses in pigs.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Lewis NS, Daly JM, Russell CA, Horton DL, Skepner E, Bryant NA, Burke DF, Rash AS, Wood JL, Chambers TM, Fouchier RA, Mumford JA, Elton DM, Smith DJ. 2011. Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. J Virol 85:12742–12749. doi:10.1128/JVI.05319-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

