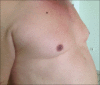
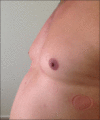
Nebivolol-induced gynecomastia
- PMID: 26312003
- PMCID: PMC4544141
- DOI: 10.4103/0976-500X.162009
Nebivolol-induced gynecomastia
Abstract
Adverse drug reactions play a substantial role in the etiology of gynecomastia. Gynecomastia as an adverse drug reaction, related to some cardiovascular drugs, has been reported in literature. Nebivolol is a third generation beta-blocker, and gynecomastia as an adverse effect on the consumption of this drug has not been reported in any article yet. We herein present the case of a 42-year-old male, who developed bilateral gynecomastia following nebivolol use and complete regression after discontinuation of nebivolol. Other reasons causing gynecomastia were excluded. Discontinuation of the responsible drug is quite sufficient with regard to the treatment of drug-induced gynecomastia, without any pharmacological or surgical treatment.
Keywords: Antihypertensive therapy; drug-induced gynecomastia; nebivolol.
Conflict of interest statement
Figures
References
-
- Donegan WL. Common benign contitions of the breast. Gynecomastia. In: Donegan WL, Spratt JS Jr, editors. Cancer of the Breast. London: W.B. Saunders; 1995. pp. 100–3.
-
- Braunstein GD. Gynecomastia. N Engl J Med. 1993;328:490–5. - PubMed
-
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method of estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. - PubMed
-
- Carlson HE. Gynecomastia. N Engl J Med. 1980;303:795–9. - PubMed
-
- Thomson DF, Carter JR. Drug-induced gynecomastia. Pharmacotherapy. 1993;13:37–45. - PubMed
Publication types
LinkOut - more resources
Full Text Sources