Key fibrogenic mediators: old players. Renin-angiotensin system
- PMID: 26312151
- PMCID: PMC4536968
- DOI: 10.1038/kisup.2014.11
Key fibrogenic mediators: old players. Renin-angiotensin system
Abstract
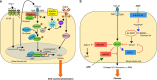
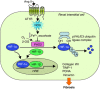
Interstitial fibrosis represents the final common pathway of any form of progressive renal disease. The severity of tubular interstitial damage is highly correlated to the degree of decline of renal function, even better than the glomerular lesions do. Angiotensin II (Ang II), the main effector of the renin-angiotensin system, is a critical promoter of fibrogenesis. It represents a nexus among glomerular capillary hypertension, barrier dysfunction, and renal tubular injury caused by abnormally filtered proteins. Transforming growth factor (TGF)-β1 and reactive oxygen species (ROS) are the key mediators of the pro-fibrotic effect of Ang II causing apoptosis and epithelial-to-mesenchymal transition of the renal tubular epithelium. Recent studies link fibrosis to changes of microRNA (miRNA) modulated by Ang II through TGF-β1, unraveling that antifibrotic action of Ang II antagonism is attributable to epigenetic control of fibrosis-associated genes. Other mechanisms of Ang II-induced fibrosis include ROS-dependent activation of hypoxia-inducible factor-1. Finally, Ang II via angiotensin type 1 receptor regulates the activation and transdifferentiation of pericytes and fibrocytes into scar-forming myofibroblasts. Detachment and phenotypic changes of the former can lead to the loss of peritubular capillaries and also contribute to hypoxia-dependent fibrosis.
Keywords: angiotensin II; fibrosis; hypoxia-inducible factor; microRNA; reactive oxygen species; transforming growth factor-β.
Figures
References
-
- Jones RH, Hayakawa H, Mackay JD, et al. Progression of diabetic nephropathy. Lancet. 1979;1:1105–1106. - PubMed
-
- Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. - PubMed
-
- Bohrer MP, Deen WM, Robertson CR, et al. Mechanism of angiotensin II-induced proteinuria in the rat. Am J Physiol. 1977;233:F13–F21. - PubMed
-
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

