Model-Based Once-Daily Darunavir/Ritonavir Dosing Recommendations in Pediatric HIV-1-Infected Patients Aged ≥3 to <12 Years
- PMID: 26312164
- PMCID: PMC4544054
- DOI: 10.1002/psp4.44
Model-Based Once-Daily Darunavir/Ritonavir Dosing Recommendations in Pediatric HIV-1-Infected Patients Aged ≥3 to <12 Years
Abstract
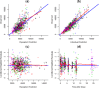
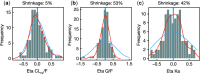
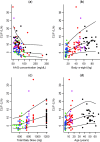
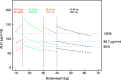
An existing population pharmacokinetic model of darunavir in adults was updated using pediatric data from two studies evaluating weight-based, once-daily dosing of darunavir/ritonavir (ARIEL, NCT00919854 and DIONE, NCT00915655). The model was then used to provide once-daily dosing recommendations for darunavir/ritonavir in pediatric patients aged ≥3 to <12 years. The final model comprised two compartments with first-order absorption and apparent clearance dependent on the concentration of α1-acid glycoprotein. The recommended darunavir/ritonavir once-daily dosing regimens in children aged ≥3 to <12 years are: 35/7 mg/kg from 10 to <15 kg, 600/100 mg from 15 to <30 kg, 675/100 mg from 30 to <40 kg, and 800/100 mg for ≥40 kg. These doses should result in exposures similar to the adult exposure after treatment with darunavir/ritonavir 800/100 mg once daily, while minimizing pill burden and allowing a switch from suspension to tablet(s) as early as possible.
Figures
Similar articles
-
Pharmacokinetics and pharmacodynamics of boosted once-daily darunavir.J Antimicrob Chemother. 2014 Oct;69(10):2591-605. doi: 10.1093/jac/dku193. Epub 2014 Jun 20. J Antimicrob Chemother. 2014. PMID: 24951533 Review.
-
Population pharmacokinetic modelling and evaluation of different dosage regimens for darunavir and ritonavir in HIV-infected individuals.J Antimicrob Chemother. 2014 Sep;69(9):2489-98. doi: 10.1093/jac/dku131. Epub 2014 May 12. J Antimicrob Chemother. 2014. PMID: 24821595
-
Evaluating darunavir/ritonavir dosing regimens for HIV-positive pregnant women using semi-mechanistic pharmacokinetic modelling.J Antimicrob Chemother. 2019 May 1;74(5):1348-1356. doi: 10.1093/jac/dky567. J Antimicrob Chemother. 2019. PMID: 30715324 Free PMC article.
-
Simultaneous pharmacogenetics-based population pharmacokinetic analysis of darunavir and ritonavir in HIV-infected patients.Clin Pharmacokinet. 2013 Jul;52(7):543-53. doi: 10.1007/s40262-013-0057-6. Clin Pharmacokinet. 2013. PMID: 23494984 Clinical Trial.
-
Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naïve and -experienced patients.HIV Clin Trials. 2008 Nov-Dec;9(6):418-27. doi: 10.1310/hct0906-418. HIV Clin Trials. 2008. PMID: 19203907 Review.
Cited by
-
Model-Based Assessment of Alternative Study Designs in Pediatric Trials. Part I: Frequentist Approaches.CPT Pharmacometrics Syst Pharmacol. 2016 Jun;5(6):305-12. doi: 10.1002/psp4.12083. Epub 2016 Jun 1. CPT Pharmacometrics Syst Pharmacol. 2016. PMID: 27300083 Free PMC article.
-
Simultaneous pharmacokinetic modeling of unbound and total darunavir with ritonavir in adolescents: a substudy of the SMILE trial.Antimicrob Agents Chemother. 2024 Feb 7;68(2):e0100423. doi: 10.1128/aac.01004-23. Epub 2023 Dec 11. Antimicrob Agents Chemother. 2024. PMID: 38092664 Free PMC article.
-
Pharmacokinetics of once-daily darunavir/ritonavir in second-line treatment in African children with HIV.J Antimicrob Chemother. 2024 Nov 4;79(11):2990-2998. doi: 10.1093/jac/dkae319. J Antimicrob Chemother. 2024. PMID: 39302766 Free PMC article. Clinical Trial.
-
Optimizing Pediatric Dosing Recommendations and Treatment Management of Antiretroviral Drugs Using Therapeutic Drug Monitoring Data in Children Living With HIV.Ther Drug Monit. 2019 Aug;41(4):431-443. doi: 10.1097/FTD.0000000000000637. Ther Drug Monit. 2019. PMID: 31008997 Free PMC article. Review.
-
Exploration of Reduced Doses and Short-Cycle Therapy for Darunavir/Cobicistat in Patients with HIV Using Population Pharmacokinetic Modeling and Simulations.Clin Pharmacokinet. 2021 Feb;60(2):177-189. doi: 10.1007/s40262-020-00920-z. Clin Pharmacokinet. 2021. PMID: 32696441 Free PMC article.
References
-
- DHHS. 2014. Guidelines for the use of antiretroviral agents in pediatric HIV infection. < http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf > ( ). Accessed 19 November 2014.
-
- Clotet B, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. - PubMed
-
- Madruga JV, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomized controlled phase III trial. Lancet. 2007;370:49–58. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

