Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both?
- PMID: 26312653
- PMCID: PMC4955369
- DOI: 10.1080/19336918.2015.1059563
Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both?
Abstract
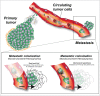
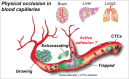
Metastasis is the end product of a multistep process where cancer cells disseminate and home themselves in distant organs. Tumor cell extravasation is a rare, inefficient and transient event in nature and makes its studies very difficult. Noteworthy, little is known about how cancer cells arrest, adhere and pass through the endothelium of capillaries. Moreover, the key events driving metastatic growth in specific organs are not well understood. Thus, although metastasis is the leading cause of cancer-related death, how cancer cells acquire their abilities to colonize distant organs and why they do so in specific locations remain central questions in the understanding of this deadly disease. In this review, we would like to confront 2 concepts explaining the efficiency and location of metastatic secondary tumors. While the "seed and soil" hypothesis states that metastasis occurs at sites where the local microenvironment is favorable, the "mechanical" concept argues that metastatic seeding occurs at sites of optimal flow patterns. In addition, recent evidence suggests that the primary event driving tumor cell arrest before extravasation is mostly controlled by blood circulation patterns as well as mechanical cues during the process of extravasation. In conclusion, the organ tropism displayed by cancer cells during metastatic colonization is a multi-step process, which is regulated by the delivery and survival of circulating tumor cells (CTCs) through blood circulation, the ability of these CTCs to adhere and cross the physical barrier imposed by the endothelium and finally by the suitability of the soil to favor growth of secondary tumors.
Keywords: extravasation, metastasis, microenvironment, biomechanics, blood flow, circulating tumor cell.
Figures

References
-
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147:275-92; PMID:22000009; http://dx.doi.org/10.1016/j.cell.2011.09.024 - DOI - PMC - PubMed
-
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell 2006; 127:679-95; PMID:17110329; http://dx.doi.org/10.1016/j.cell.2006.11.001 - DOI - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013 - DOI - PubMed
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989; 8:98-101; PMID:2673568 - PubMed
-
- Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 1992; 10:191-9; PMID:1582089; http://dx.doi.org/10.1007/BF00132751 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
