Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence
- PMID: 26313138
- PMCID: PMC5457709
- DOI: 10.1021/acs.chemrev.5b00321
Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence
Abstract
Conflict of interest statement
The authors declare the following competing financial interest(s): C.A.M. is a cofounder of AuraSense, LLC, a startup biotechnology company that licenses the NanoFlare technology from Northwestern University.
Figures





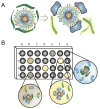






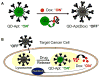
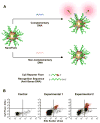


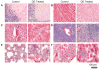



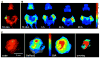
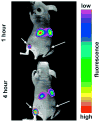


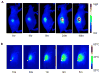
References
-
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer Treatment and Survivorship Statistics, 2014. Ca-Cancer J Clin. 2014;64:252–271. - PubMed
-
- World Health Organization. 2014 www.who.int.
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. Ca-Cancer J Clin. 2014;64:9–29. - PubMed
-
- Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

