A practical, automated synthesis of meta-[(18)F]fluorobenzylguanidine for clinical use
- PMID: 26313342
- PMCID: PMC4651823
- DOI: 10.1021/acschemneuro.5b00202
A practical, automated synthesis of meta-[(18)F]fluorobenzylguanidine for clinical use
Abstract
Many neuroendocrine tumors, such as neuroblastoma (NB), arise from neural crest cells of the sympathetic nervous system. This nerve-like phenotype has been exploited for functional imaging using radioactive probes originally designed for neuronal and adrenal medullary applications. NB imaging with meta-[(123)I]iodobenzylguanidine ([(123)I]MIBG) is limited by the emissions of (123)I, which lead to poor image resolution and challenges in quantification of its accumulation in tumors. meta-[(18)F]Fluorobenzylguanidine ([(18)F]MFBG) is a promising alternative to [(123)I]MIBG that could change the standard of practice for imaging neuroendocrine tumors, but interest in this PET radiotracer has suffered due to its complex and inefficient radiosynthesis. Here we report a two-step, automated method for the routine production of [(18)F]MFBG by thermolysis of a diaryliodonium fluoride and subsequent acid deprotection. The synthesis was adapted for use on a commercially available synthesizer for routine production. Full characterization of [(18)F]MFBG produced by this route demonstrated the tracer's suitability for human use. [(18)F]MFBG was prepared in almost 3-fold higher yield than previously reported (31% corrected to end of bombardment, n = 9) in a synthesis time of 56 min with >99.9% radiochemical purity. Other than pH adjustment and dilution of the final product, no reformulation was necessary after purification. This method permits the automated production of multidose batches of clinical grade [(18)F]MFBG. Moreover, if ongoing clinical imaging trials of [(18)F]MFBG are successful, this methodology is suitable for rapid commercialization and can be easily adapted for use on most commercial automated radiosynthesis equipment.
Keywords: [18F]MFBG; diaryliodonium salts; fluorine-18; meta-[18F]Fluorobenzylguanidine; positron emission tomography.
Figures





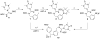
References
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Sharp SE, Gelfand MJ, Shulkin BL. PET/CT in the evaluation of neuroblastoma. PET Clinics. 2009;3:551–561. - PubMed
-
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and Adolescent Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:83–103. - PubMed
-
- Brodeur GM, Hogarty MD, Mosse YP, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 6. Lippincott Williams & Wilkins; Philadelphia: 2011. pp. 887–922.
-
- Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

