Role of adult hippocampal neurogenesis in persistent pain
- PMID: 26313405
- PMCID: PMC4858177
- DOI: 10.1097/j.pain.0000000000000332
Role of adult hippocampal neurogenesis in persistent pain
Abstract
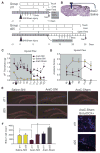
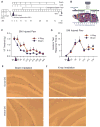
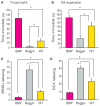
The full role of adult hippocampal neurogenesis (AHN) remains to be determined, yet it is implicated in learning and emotional functions, and is disrupted in negative mood disorders. Recent evidence indicates that AHN is decreased in persistent pain consistent with the idea that chronic pain is a major stressor, associated with negative moods and abnormal memories. Yet, the role of AHN in development of persistent pain has remained unexplored. In this study, we test the influence of AHN in postinjury inflammatory and neuropathic persistent pain-like behaviors by manipulating neurogenesis: pharmacologically through intracerebroventricular infusion of the antimitotic AraC; ablation of AHN by x-irradiation; and using transgenic mice with increased or decreased AHN. Downregulating neurogenesis reversibly diminished or blocked persistent pain; oppositely, upregulating neurogenesis led to prolonged persistent pain. Moreover, we could dissociate negative mood from persistent pain. These results suggest that AHN-mediated hippocampal learning mechanisms are involved in the emergence of persistent pain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
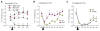
Comment in
-
Hippocampal neurogenesis: does it relieve or worsen chronic pain?Pain. 2016 Feb;157(2):506-507. doi: 10.1097/j.pain.0000000000000418. Pain. 2016. PMID: 26797509 No abstract available.
-
Reply.Pain. 2016 Feb;157(2):508-509. doi: 10.1097/j.pain.0000000000000419. Pain. 2016. PMID: 26797510 Free PMC article. No abstract available.
References
-
- Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saade N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol. 2011;228:30–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

