Anti-NMDA Receptor Encephalitis in the Polar Bear (Ursus maritimus) Knut
- PMID: 26313569
- PMCID: PMC4551079
- DOI: 10.1038/srep12805
Anti-NMDA Receptor Encephalitis in the Polar Bear (Ursus maritimus) Knut
Abstract
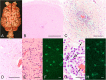
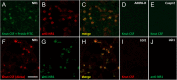
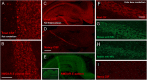
Knut the polar bear of the Berlin Zoological Garden drowned in 2011 following seizures and was diagnosed as having suffered encephalitis of unknown etiology after exhaustive pathogen screening. Using the diagnostic criteria applied to human patients, we demonstrate that Knut's encephalitis is almost identical to anti-NMDA receptor encephalitis which is a severe autoimmune disease representing the most common non-infectious encephalitis in humans. High concentrations of antibodies specific against the NR1 subunit of the NMDA receptor were detected in Knut's cerebrospinal fluid. Histological examination demonstrated very similar patterns of plasma cell infiltration and minimal neuronal loss in affected brain areas. We conclude that Knut suffered anti-NMDA receptor encephalitis making his the first reported non-human case of this treatable disease. The results suggest that anti-NMDA receptor encephalitis may be a disease of broad relevance to mammals that until now has remained undiagnosed.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Szentiks C. A. et al.. Polar bear encephalitis: establishment of a comprehensive nextgeneration pathogen analysis pipeline for captive and free-living wildlife. J Comp Pathol 150, 474–488 (2014). - PubMed
-
- Prüss H. et al.. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 75, 1735–1739 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

