HLA class I is most tightly linked to levels of tapasin compared with other antigen-processing proteins in glioblastoma
- PMID: 26313662
- PMCID: PMC4578088
- DOI: 10.1038/bjc.2015.297
HLA class I is most tightly linked to levels of tapasin compared with other antigen-processing proteins in glioblastoma
Erratum in
-
HLA class I is most tightly linked to levels of tapasin compared with other antigen-processing proteins in glioblastoma.Br J Cancer. 2015 Dec 1;113(11):1640. doi: 10.1038/bjc.2015.387. Br J Cancer. 2015. PMID: 26625215 Free PMC article. No abstract available.
Abstract
Background: Tumour cells can evade the immune system by dysregulation of human leukocyte antigens (HLA-I). Low quantity and/or altered quality of HLA-I cell surface expression is the result of either HLA-I alterations or dysregulations of proteins of the antigen-processing machinery (APM). Tapasin is an APM protein dedicated to the maturation of HLA-I and dysregulation of tapasin has been linked to higher malignancy in several different tumours.
Methods: We studied the expression of APM components and HLA-I, as well as HLA-I tapasin-dependency profiles in glioblastoma tissues and corresponding cell lines.
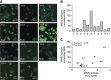
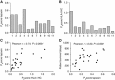
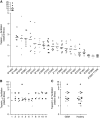
Results: Tapasin displayed the strongest correlation to HLA-I heavy chain but also clustered with β2-microglobulin, transporter associated with antigen processing (TAP) and LMP. Moreover, tapasin also correlated to survival of glioblastoma patients. Some APM components, for example, TAP1/TAP2 and LMP2/LMP7, showed variable but coordinated expression, whereas ERAP1/ERAP2 displayed an imbalanced expression pattern. Furthermore, analysis of HLA-I profiles revealed variable tapasin dependence of HLA-I allomorphs in glioblastoma patients.
Conclusions: Expression of APM proteins is highly variable between glioblastomas. Tapasin stands out as the APM component strongest correlated to HLA-I expression and we proved that HLA-I profiles in glioblastoma patients include tapasin-dependent allomorphs. The level of tapasin was also correlated with patient survival time. Our results support the need for individualisation of immunotherapy protocols.
Figures



References
-
- Aladin F, Lautscham G, Humphries E, Coulson J, Blake N (2007) Targeting tumour cells with defects in the MHC Class I antigen processing pathway with CD8+ T cells specific for hydrophobic TAP- and Tapasin-independent peptides: the requirement for directed access into the ER. Cancer Immunol Immunother 56(8): 1143–1152. - PMC - PubMed
-
- Bandoh N, Ogino T, Katayama A, Takahara M, Katada A, Hayashi T, Harabuchi Y (2010) HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep 23(4): 933–939. - PubMed
-
- Barber LD, Howarth M, Bowness P, Elliott T (2001) The quantity of naturally processed peptides stably bound by HLA-A*0201 is significantly reduced in the absence of tapasin. Tissue Antigens 58(6): 363–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

