Treatment of inflammatory myopathy: emerging therapies and therapeutic targets
- PMID: 26313852
- PMCID: PMC4864492
- DOI: 10.1586/1744666X.2015.1082908
Treatment of inflammatory myopathy: emerging therapies and therapeutic targets
Abstract
Despite the lack of placebo-controlled trials, glucocorticoids are considered the mainstay of initial treatment for idiopathic inflammatory myopathy and myositis-associated interstitial lung disease. Glucocorticoid-sparing agents are often given concomitantly with other immunosuppressive agents, particularly in patients with moderate or severe disease. First-line conventional immunosuppressive drugs include either methotrexate or azathioprine, and when they fail, more aggressive therapy includes mycophenolate mofetil, tacrolimus or cyclosporine, intravenous immunoglobulin, rituximab, or cyclophosphamide, used alone or in various combinations. Further investigations are required to assess the role of more novel therapies in the treatment of myositis and myositis-associated interstitial lung disease.
Keywords: dermatomyositis; idiopathic inflammatory myopathy; myositis; polymyositis; treatment.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
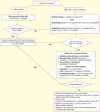
References
-
-
Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314–324.• This manuscript summarizes the results of the largest randomized, double-blind, controlled clinical trial in idiopathic inflammatory myopathy (the Rituximab in Myositis [RIM] trial)
-
-
- Bunch TW, Worthington JW, Combs JJ, et al. Azathioprine with prednisone for polymyositis A controlled, clinical trial. Ann Intern Med. 1980;92(3):365–369. - PubMed
-
- Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329(27):1993–2000. - PubMed
-
- Miller FW, Leitman SF, Cronin ME, et al. Controlled trial of plasma exchange and leukapheresis in polymyositis and dermatomyositis. N Engl J Med. 1992;326(21):1380–1384. - PubMed
-
- Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore) 2005;84(4):231–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
