Shedding of Infectious Borna Disease Virus-1 in Living Bicolored White-Toothed Shrews
- PMID: 26313904
- PMCID: PMC4552160
- DOI: 10.1371/journal.pone.0137018
Shedding of Infectious Borna Disease Virus-1 in Living Bicolored White-Toothed Shrews
Abstract
Background: Many RNA viruses arise from animal reservoirs, namely bats, rodents and insectivores but mechanisms of virus maintenance and transmission still need to be addressed. The bicolored white-toothed shrew (Crocidura leucodon) has recently been identified as reservoir of the neurotropic Borna disease virus 1 (BoDV-1).
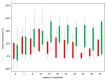
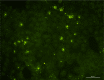
Principal findings: Six out of eleven wild living bicoloured white-toothed shrews were trapped and revealed to be naturally infected with BoDV-1. All shrews were monitored in captivity in a long-term study over a time period up to 600 days that differed between the individual shrews. Interestingly, all six animals showed an asymptomatic course of infection despite virus shedding via various routes indicating a highly adapted host-pathogen interaction. Infectious virus and viral RNA were demonstrated in saliva, urine, skin swabs, lacrimal fluid and faeces, both during the first 8 weeks of the investigation period and for long time shedding after more than 250 days in captivity.
Conclusions: The various ways of shedding ensure successful virus maintenance in the reservoir population but also transmission to accidental hosts such as horses and sheep. Naturally BoDV-1-infected living shrews serve as excellent tool to unravel host and pathogen factors responsible for persistent viral co-existence in reservoir species while maintaining their physiological integrity despite high viral load in many organ systems.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

