HMGB1 in the pathogenesis of ultraviolet-induced ocular surface inflammation
- PMID: 26313914
- PMCID: PMC4558494
- DOI: 10.1038/cddis.2015.199
HMGB1 in the pathogenesis of ultraviolet-induced ocular surface inflammation
Abstract
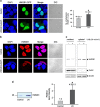
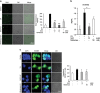
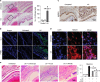
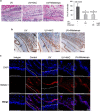
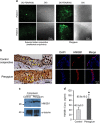
High-mobility group box 1 (HMGB1) functions as a transcription-enhancing nuclear protein as well as a crucial cytokine that regulates inflammation. This study demonstrated that secretion of HMGB1 due to ultraviolet (UV) radiation inducing ocular surface inflammation-mediated reactive oxygen species (ROS) production. After treating conjunctival epithelial cells with UV radiation, HMGB1 was translocated from the nucleus to the cytoplasm and then eventually to the extracellular space. HMGB1 played a crucial role in UV-induced conjunctival neutrophil infiltration, which subsided when mice were pretreated with the HMGB1 inhibitors soluble receptor for advanced glycation endproducts (sRAGEs) and HMGB1 A box protein. In case of using ROS quencher, there was decrease in UV-induced HMGB1 secretion in conjunctival epithelial cells and mice. Considering that UV-induced chronic inflammation causes ocular surface change as pterygium, we have confirmed high HMGB1 translocation and ROS expression in human pterygium. Our findings therefore revealed a previously unknown mechanism of UV-induced ocular inflammation related to ROS and HMGB1 suggesting a new medical therapeutic target.
Figures
References
-
- Ueda T, Yoshida M. HMGB proteins and transcriptional regulation. Biochim Biophys Acta. 2010;1799:114–118. - PubMed
-
- Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. - PubMed
-
- Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182:5800–5809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

