Cysteine-Mediated Redox Regulation of Cell Signaling in Chondrocytes Stimulated With Fibronectin Fragments
- PMID: 26314228
- PMCID: PMC4849859
- DOI: 10.1002/art.39326
Cysteine-Mediated Redox Regulation of Cell Signaling in Chondrocytes Stimulated With Fibronectin Fragments
Abstract
Objective: Oxidative posttranslational modifications of intracellular proteins can potentially regulate signaling pathways relevant to cartilage destruction in arthritis. In this study, oxidation of cysteine residues to form sulfenic acid (S-sulfenylation) was examined in osteoarthritic (OA) chondrocytes and investigated in normal chondrocytes as a mechanism by which fragments of fibronectin (FN-f) stimulate chondrocyte catabolic signaling.
Methods: Chondrocytes isolated from OA and normal human articular cartilage were analyzed using analogs of dimedone that specifically and irreversibly react with protein S-sulfenylated cysteines. Global S-sulfenylation was measured in cell lysates with and without FN-f stimulation by immunoblotting and in fixed cells by confocal microscopy. S-sulfenylation in specific proteins was identified by mass spectroscopy and confirmed by immunoblotting. Src activity was measured in live cells using a fluorescence resonance energy transfer biosensor.
Results: Proteins in chondrocytes isolated from OA cartilage were found to have elevated basal levels of S-sulfenylation relative to those of chondrocytes from normal cartilage. Treatment of normal chondrocytes with FN-f induced increased levels of S-sulfenylation in multiple proteins, including the tyrosine kinase Src. FN-f treatment also increased the levels of Src activity. Pretreatment with dimedone to alter S-sulfenylation function or with Src kinase inhibitors inhibited FN-f-induced production of matrix metalloproteinase 13.
Conclusion: These results demonstrate for the first time the presence of oxidative posttranslational modification of proteins in human articular chondrocytes by S-sulfenylation. Due to the ability to regulate the activity of a number of cell signaling pathways, including catabolic mediators induced by fibronectin fragments, S-sulfenylation may contribute to cartilage destruction in OA and warrants further investigation.
© 2016, American College of Rheumatology.
Figures


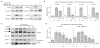


References
-
- Dunn JD, Pill MW. A claims-based view of health care charges and utilization for commercially insured patients with osteoarthritis. Manag Care. 2009;18:44–50. - PubMed
-
- Ding L, Guo D, Homandberg GA. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-Src, NF-κB and protein kinase Cδ. Osteoarthritis Cartilage. 2009;17:1385–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

