Injectable silk-based biomaterials for cervical tissue augmentation: an in vitro study
- PMID: 26314518
- PMCID: PMC4698015
- DOI: 10.1016/j.ajog.2015.08.046
Injectable silk-based biomaterials for cervical tissue augmentation: an in vitro study
Abstract
Background: Cerclage therapy is an important treatment option for preterm birth prevention. Several patient populations benefit from cerclage therapy including patients with a classic history of cervical insufficiency; patients who present with advanced cervical dilation prior to viability; and patients with a history of preterm birth and cervical shortening. Although cerclage is an effective treatment option in some patients, it can be associated with limited efficacy and procedure complications. Development of an alternative to cerclage therapy would be an important clinical development. Here we report on an injectable, silk protein-based biomaterial for cervical tissue augmentation. The rationale for the development of an injectable biomaterial is to restore the native properties of cervical tissue. While cerclage provides support to the tissue, it does not address excessive tissue softening, which is a central feature of the pathogenesis of cervical insufficiency. Silk protein-based hydrogels, which are biocompatible and naturally degrade in vivo, are suggested as a platform for restoring the native properties of cervical tissue and improving cervical function.
Objective: We sought to study the properties of an injectable, silk-based biomaterial for potential use as an alternative treatment for cervical insufficiency. These biomaterials were evaluated for mechanical tunability, biocompatibility, facile injection, and in vitro degradation.
Study design: Silk protein solutions were cross-linked by an enzyme catalyzed reaction to form elastic biomaterials. Biomaterials were formulated to match the native physical properties of cervical tissue during pregnancy. The cell compatibility of the materials was assessed in vitro using cervical fibroblasts, and biodegradation was evaluated using concentrated protease solution. Tissue augmentation or bulking was demonstrated using human cervical tissue from nonpregnant hysterectomy specimens. Mechanical compression tests measured the tissue stiffness as a function of the volume of injected biomaterial.
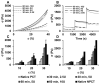
Results: Silk protein concentration, molecular weight, and concentration of cross-linking agent were varied to generate biomaterials that functioned from hard gels to viscous fluids. Biomaterials that matched the mechanical features of cervical tissues were chosen for further study. Cervical fibroblasts cultured on these biomaterials were proliferative and metabolically active over 6 days. Biomaterials were degraded in protease solution, with rate of mass loss dependent on silk protein molecular weight. Injection of cervical tissue samples with 100 μL of the biomaterial resulted in a significant volume increase (22.6% ± 8.8%, P < .001) with no significant change in tissue stiffness.
Conclusion: Cytocompatible, enzyme cross-linked silk protein biomaterials show promise as a tissue bulking agent. The biomaterials were formulated to match the native mechanical properties of human cervical tissue. These biomaterials should be explored further as a possible alternative to cerclage for providing support to the cervix during pregnancy.
Keywords: cervical shortening; cervical tissue bulking; hydrogels; preterm birth; silk protein.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- O’Connor A, Wilson C, Fielder A. Ophthalmological problems associated with preterm birth. Eye. 2007;21:1254–60. DOI: 10.1038/sj.eye.6702838. - PubMed
-
- Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. DOI: 10.1056/NEJMoa1005372. - PubMed
-
- Doyle LW. Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics. 2001;108(1):134–41. DOI: 10.1542/peds.108.1.134. - PubMed
-
- Greenough A. Long term respiratory outcomes of very premature birth (<32 weeks) Semin Fetal Neonatal Med. 2012;17(2):73–6. DOI: 10.1016/j.siny.2012.01.009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

