Phenotypic, Functional, and Gene Expression Profiling of Peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127(low) Treg Cells in Patients With Chronic Rheumatoid Arthritis
- PMID: 26314565
- PMCID: PMC4832388
- DOI: 10.1002/art.39408
Phenotypic, Functional, and Gene Expression Profiling of Peripheral CD45RA+ and CD45RO+ CD4+CD25+CD127(low) Treg Cells in Patients With Chronic Rheumatoid Arthritis
Abstract
Objective: Conflicting evidence exists regarding the suppressive capacity of Treg cells in the peripheral blood (PB) of patients with rheumatoid arthritis (RA). The aim of this study was to determine whether Treg cells are intrinsically defective in RA.
Methods: Using a range of assays on PB samples from patients with chronic RA and healthy controls, CD3+CD4+CD25+CD127(low) Treg cells from the CD45RO+ or CD45RA+ T cell compartments were analyzed for phenotype, cytokine expression (ex vivo and after in vitro stimulation), suppression of Teff cell proliferation and cytokine production, suppression of monocyte-derived cytokine/chemokine production, and gene expression profiles.
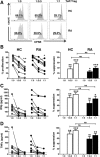
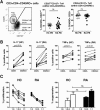
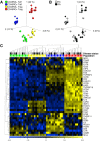
Results: No differences between RA patients and healthy controls were observed with regard to the frequency of Treg cells, ex vivo phenotype (CD4, CD25, CD127, CD39, or CD161), or proinflammatory cytokine profile (interleukin-17 [IL-17], interferon-γ [IFNγ], or tumor necrosis factor [TNF]). FoxP3 expression was slightly increased in Treg cells from RA patients. The ability of Treg cells to suppress the proliferation of T cells or the production of cytokines (IFNγ or TNF) upon coculture with autologous CD45RO+ Teff cells and monocytes was not significantly different between RA patients and healthy controls. In PB samples from some RA patients, CD45RO+ Treg cells showed an impaired ability to suppress the production of certain cytokines/chemokines (IL-1β, IL-1 receptor antagonist, IL-7, CCL3, or CCL4) by autologous lipopolysaccharide-activated monocytes. However, this was not observed in all patients, and other cytokines/chemokines (TNF, IL-6, IL-8, IL-12, IL-15, or CCL5) were generally suppressed. Finally, gene expression profiling of CD45RA+ or CD45RO+ Treg cells from the PB revealed no statistically significant differences between RA patients and healthy controls.
Conclusion: Our findings indicate that there is no global defect in either CD45RO+ or CD45RA+ Treg cells in the PB of patients with chronic RA.
© 2016 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures


References
-
- Cao D, Malmstrom V, Baecher‐Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 2003;33:215–23. - PubMed
-
- Van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 2004;50:2775–85. - PubMed
-
- Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine‐suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol 2005;62:312–7. - PubMed
-
- Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 2006;45:1210–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

