14-3-3 inhibition promotes dopaminergic neuron loss and 14-3-3θ overexpression promotes recovery in the MPTP mouse model of Parkinson's disease
- PMID: 26314634
- PMCID: PMC4594956
- DOI: 10.1016/j.neuroscience.2015.08.042
14-3-3 inhibition promotes dopaminergic neuron loss and 14-3-3θ overexpression promotes recovery in the MPTP mouse model of Parkinson's disease
Abstract
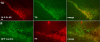
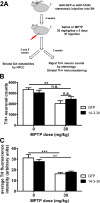
14-3-3s are a highly conserved protein family that plays important roles in cell survival and interact with several proteins implicated in Parkinson's disease (PD). Disruption of 14-3-3 expression and function has been implicated in the pathogenesis of PD. We have previously shown that increasing the expression level of 14-3-3θ is protective against rotenone and 1-methyl-4-phenylpyridinium (MPP(+)) in cultured cells. Here, we extend our studies to examine the effects of 14-3-3s in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. We first investigated whether targeted nigral 14-3-3θ overexpression mediated by adeno-associated virus offers neuroprotection against MPTP-induced toxicity. 14-3-3θ overexpression using this approach did not reduce MPTP-induced dopaminergic cell loss in the substantia nigra nor the depletion of dopamine (DA) and its metabolites in the striatum at three weeks after MPTP administration. However, 14-3-3θ-overexpressing mice showed a later partial recovery in striatal DA metabolites at eight weeks after MPTP administration compared to controls, suggesting that 14-3-3θ overexpression may help in the functional recovery of those dopaminergic neurons that survive. Conversely, we investigated whether disrupting 14-3-3 function in transgenic mice expressing the pan 14-3-3 inhibitor difopein exacerbates MPTP-induced toxicity. We found that difopein expression promoted dopaminergic cell loss in response to MPTP treatment. Together, these findings suggest that 14-3-3θ overexpression promotes recovery of DA metabolites whereas 14-3-3 inhibition exacerbates neuron loss in the MPTP mouse model of PD.
Keywords: 14-3-3s; MPTP; Parkinson’s disease; adeno-associated virus; dopamine; neurodegeneration.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
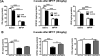



References
-
- Berg D, Riess O, Bornemann A. Specification of 14-3-3 proteins in Lewy bodies. Ann Neurol. 2003;54:135. - PubMed
-
- Brunelli L, Cieslik KA, Alcorn JL, Vatta M, Baldini A. Peroxisome proliferator-activated receptor-delta upregulates 14-3-3 epsilon in human endothelial cells via CCAAT/enhancer binding protein-beta. Circ Res. 2007;100:e59–71. - PubMed
-
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117:1875–1884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

