Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review
- PMID: 26314872
- PMCID: PMC4757311
- DOI: 10.1177/1971400915602803
Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review
Abstract
Background: Flow-diverter devices (FDDs) are new-generation stents placed in the parent artery at the level of the aneurysm neck to disrupt the intra-aneurysmal flow thus favoring intra-aneurysmal thrombosis.
Objective: The objective of this review article is to define the indication and results of the treatment of intracranial aneurysms by FDD, reviewing 18 studies of endovascular treatment by FDDs for a total of 1704 aneurysms in 1483 patients.
Methods: The medical literature on FDDs for intracranial aneurysms was reviewed from 2009 to December 2014. The keywords used were: "intracranial aneurysms," "brain aneurysms," "flow diverter," "pipeline embolization device," "silk flow diverter," "surpass flow diverter" and "FRED flow diverter."
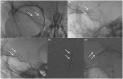
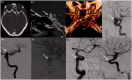
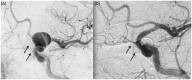
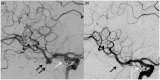
Results: The use of these stents is advisable mainly for unruptured aneurysms, particularly those located at the internal carotid artery or vertebral and basilar arteries, for fusiform and dissecting aneurysms and for saccular aneurysms with large necks and low dome-to-neck ratio. The rate of aneurysm occlusion progressively increases during follow-up (81.5% overall rate in this review). The non-negligible rate of ischemic (mean 4.1%) and hemorrhagic (mean 2.9%) complications, the neurological morbidity (mean 3.5%) and the reported mortality (mean 3.4%) are the main limits of this technique.
Conclusion: Treatment with FDDs is a feasible and effective technique for unruptured aneurysms with complex anatomy (fusiform, dissecting, large neck, bifurcation with side branches) where coiling and clipping are difficult or impossible. Patient selection is very important to avoid complications and reduce the risk of morbidity and mortality. Further studies with longer follow-up are necessary to define the rate of complete occlusion.
Keywords: Intracranial aneurysm; Silk embolization device; brain aneurysm; endovascular treatment; flow-diverter devices; pipeline embolization device; surpass embolization device.
© The Author(s) 2015.
Figures

References
-
- Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: Systematic review and meta-analysis. Neurosurgery 2013; 73: 193–199. discussion 199–200. - PubMed
-
- Brinjikji W, Mohammad HM, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. - PubMed
-
- Diaz O, Gist TL, Manjarez G, et al. Treatment of 14 intracranial aneurysms with the FRED system. J Neurointerv Surg 2014; 6: 614–617. - PubMed
-
- Kocer N, Islak C, Kizilkilic O, et al. Flow Re-direction Endoluminal Device in treatment of cerebral aneurysms: Initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. - PubMed
-
- Pereira VM, Kelly M, Vega P, et al. New Pipeline Flex device: Initial experience and technical nuances. J Neurointerv Surg. Epub ahead of print 3 October 2014. DOI: 10.1136/neurintsurg-2014-011347. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

