Concentration-Dependent Diversifcation Effects of Free Cholesterol Loading on Macrophage Viability and Polarization
- PMID: 26314949
- PMCID: PMC4752209
- DOI: 10.1159/000430365
Concentration-Dependent Diversifcation Effects of Free Cholesterol Loading on Macrophage Viability and Polarization
Abstract
Background/aims: The accumulation of free cholesterol in atherosclerotic lesions has been well documented in both animals and humans. In studying the relevance of free cholesterol buildup in atherosclerosis, contradictory results have been generated, indicating that free cholesterol produces both pro- and anti-atherosclerosis effects in macrophages. This inconsistency might stem from the examination of only select concentrations of free cholesterol. In the present study, we sought to investigate the implication of excess free cholesterol loading in the pathophysiology of atherosclerosis across a broad concentration range from (in µg/ml) 0 to 60.
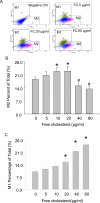
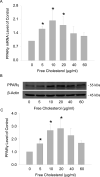
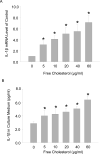
Methods: Macrophage viability was determined by measuring formazan formation and flow cytometry viable cell counting. The polarization of M1 and M2 macrophages was differentiated by FACS (Fluorescence-Activated Cell Sorting) assay. The secretion of IL-1β in macrophage culture medium was measured by ELISA kit. Macrophage apoptosis was detected by flow cytometry using a TUNEL kit.
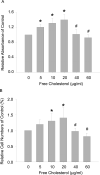
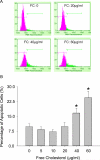
Results: Macrophage viability was increased at the treatment of lower concentrations of free cholesterol from (in µg/ml) 0 to 20, but gradually decreased at higher concentrations from 20 to 60. Lower free cholesterol loading induced anti-inflammatory M2 macrophage polarization. The activation of the PPARx03B3; (Peroxisome Proliferator-Activated Receptor gamma) nuclear factor underscored the stimulation of this M2 phenotype. Nevertheless, higher levels of free cholesterol resulted in pro-inflammatory M1 activation. Moreover, with the application of higher free cholesterol concentrations, macrophage apoptosis and secretion of the inflammatory cytokine IL-1β increased significantly.
Conclusion: These results for the first time demonstrate that free cholesterol could render concentration-dependent diversification effects on macrophage viability, polarization, apoptosis and inflammatory cytokine secretions, thereby reconciling the pros and cons of free cholesterol buildup in macrophages to the pathophysiology of atherosclerosis.
© 2015 S. Karger AG, Basel.
Figures





References
-
- Collot-Teixeira S, Martin J, Mcdermott-Roe C, Poston R, Mcgregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. - PubMed
-
- Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12:289–296. - PubMed
-
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. - PubMed
-
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
