Low doses of X-rays induce prolonged and ATM-independent persistence of γH2AX foci in human gingival mesenchymal stem cells
- PMID: 26314960
- PMCID: PMC4694989
- DOI: 10.18632/oncotarget.4739
Low doses of X-rays induce prolonged and ATM-independent persistence of γH2AX foci in human gingival mesenchymal stem cells
Abstract
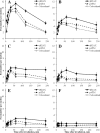
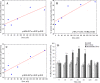
Diagnostic imaging delivering low doses of radiation often accompany human mesenchymal stem cells (MSCs)-based therapies. However, effects of low dose radiation on MSCs are poorly characterized. Here we examine patterns of phosphorylated histone H2AX (γH2AX) and phospho-S1981 ATM (pATM) foci formation in human gingiva-derived MSCs exposed to X-rays in time-course and dose-response experiments. Both γH2AX and pATM foci accumulated linearly with dose early after irradiation (5-60 min), with a maximum induction observed at 30-60 min (37 ± 3 and 32 ± 3 foci/cell/Gy for γH2AX and pATM, respectively). The number of γH2AX foci produced by intermediate doses (160 and 250 mGy) significantly decreased (40-60%) between 60 and 240 min post-irradiation, indicating rejoining of DNA double-strand breaks. In contrast, γH2AX foci produced by low doses (20-80 mGy) did not change after 60 min. The number of pATM foci between 60 and 240 min decreased down to control values in a dose-independent manner. Similar kinetics was observed for pATM foci co-localized with γH2AX foci. Collectively, our results suggest differential DNA double-strand break signaling and processing in response to low vs. intermediate doses of X-rays in human MSCs. Furthermore, mechanisms governing the prolonged persistence of γH2AX foci in these cells appear to be ATM-independent.
Keywords: DNA double-strand breaks; DNA repair; X-rays; low doses; mesenchymal stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cipriani P, Ruscitti P, Di Benedetto P, Carubbi F, Liakouli V, Berardicurti O, Ciccia F, Triolo G, Giacomelli R. Mesenchymal stromal cells and rheumatic diseases: new tools from pathogenesis to regenerative therapies. Cytotherapy. 2015;17:832–849. - PubMed
-
- Caplan AI. Mesenchymal stem cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1991;9:641–650. - PubMed
-
- Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14:516–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

