Elevated S100A9 expression in tumor stroma functions as an early recurrence marker for early-stage oral cancer patients through increased tumor cell invasion, angiogenesis, macrophage recruitment and interleukin-6 production
- PMID: 26315114
- PMCID: PMC4695068
- DOI: 10.18632/oncotarget.4951
Elevated S100A9 expression in tumor stroma functions as an early recurrence marker for early-stage oral cancer patients through increased tumor cell invasion, angiogenesis, macrophage recruitment and interleukin-6 production
Abstract
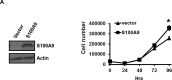
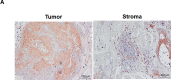
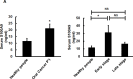
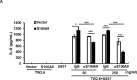
S100A9 is a calcium-binding protein with two EF-hands and frequently deregulated in several cancer types, however, with no clear role in oral cancer. In this report, the expression of S100A9 in cancer and adjacent tissues from 79 early-stage oral cancer patients was detected by immunohistochemical staining. Although S100A9 protein was present in both tumor and stromal cells, only the early-stage oral cancer patients with high stromal expression had reduced recurrence-free survival. High stromal S100A9 expression was also significantly associated with non-well differentiation and recurrence. In addition to increasing cell migration and invasion, ectopic S100A9 expression in tumor cells promoted xenograft tumorigenesis as well as the dominant expression of myeloid cell markers and pro-inflammatory IL-6. The expression of S100A9 in one stromal component, monocytes, stimulated the aggressiveness of co-cultured oral cancer cells. We also detected the elevation of serum S100A9 levels in early-stage oral cancer patients of a separate cohort of 73 oral cancer patients. The release of S100A9 protein into extracellular milieu enhanced tumor cell invasion, transendothelial monocyte migration and angiogenic activity. S100A9-mediated release of IL-6 requires the crosstalk of tumor cells with monocytes through the activation of NF-κB and STAT-3. Early-stage oral cancer patients with both high S100A9 expression and high CD68+ immune infiltrates in stroma had shortest recurrence-free survival, suggesting the use of both S100A9 and CD68 as poor prognostic markers for oral cancer. Together, both intracellular and extracellular S100A9 exerts a tumor-promoting action through the activation of oral cancer cells and their associated stroma in oral carcinogenesis.
Keywords: IL-6; S100A9; macrophages; oral cancer; recurrence.
Conflict of interest statement
All the authors in this manuscript state no conflict of interest.
Figures























References
-
- Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2006;102:67–76. - PubMed
-
- Lian Ie B, Tseng YT, Su CC, Tsai KY. Progression of precancerous lesions to oral cancer: results based on the Taiwan National Health Insurance Database. Oral Oncol. 2013;49:427–430. - PubMed
-
- Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. Journal of leukocyte biology. 2007;81:28–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

