CHO expressed recombinant human lactoferrin as an adjuvant for BCG
- PMID: 26315722
- PMCID: PMC6004602
- DOI: 10.1177/0394632015599832
CHO expressed recombinant human lactoferrin as an adjuvant for BCG
Abstract
Lactoferrin (LF), an iron binding protein with immune modulatory activities, has adjuvant activity to enhance vaccine efficacy. Tuberculosis (TB) is a pulmonary disease caused by the pathogen Mycobacterium tuberculosis (MTB). Progressive TB disease is clinically defined by damaging pulmonary pathology, a result of inflammation due to immune reactivity. The current vaccine for TB, an attenuated strain of Mycobacterium bovis, Bacillus Calmette Guerin (BCG), has only limited efficacy to prevent adult pulmonary TB. This study examines a Chinese hamster ovary (CHO) expressed recombinant human LF (rHLF) to boost efficacy of the BCG vaccine and delay early pathology post infectious challenge. C57BL/6 mice were immunized with BCG, or BCG admixed with either rHLF or bovine LF (bLF; internal control), or remained unvaccinated. Mice were then aerosol challenged with Erdman MTB. All vaccinated mice demonstrated decreased organ bacterial load up to 19 weeks post infection compared with non-vaccinated controls. Furthermore, mice receiving bLF or rHLF supplemented BCG vaccines showed a modest decrease in lung pathology developed over time, compared to the BCG vaccine alone. While mice vaccinated with BCG/rHLF demonstrated increased general lung inflammation at day 7, it occurred without noticeable increase in pro-inflammatory cytokines. At later times, decreased pathology in the rHLF groups correlated with decreased inflammatory cytokines. Splenic recall to BCG antigens showed BCG/rHLF vaccination increased production of IFN-γ, IL-6, and GM-CSF compared to naïve, BCG, and BCG/bLF groups. Analysis of T cell stimulating functions of bone marrow derived macrophages and dendritic cells treated with BCG/bLF or BCG/rHLF showed decreases in IL-10 production when co-cultured with sensitized CD4 and CD8 T cells, compared to those cultured with macrophages/dendritic cells treated with BCG without LF. These results indicate that addition of rHLF to the BCG vaccine can modulate development of host pathology early post infectious challenge, most likely through host immune regulation affecting hypersensitive responses.
Keywords: BCG; CHO; adjuvant; lactoferrin; tuberculosis.
© The Author(s) 2015.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Access to research materials may be made available upon request, conforming to rules and regulations of the National Institutes of Health, and the University of Texas-Houston Health Science Center.
Figures
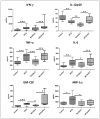






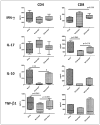

References
-
- Barreto ML, Pereira SM, Ferreira AA. BCG vaccine: Efficacy and indications for vaccination and revaccination. Jornal de Pediatria. 2006;82(Suppl 3):45–54. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Journal of the American Medical Association. 1994;271(279):698–702. - PubMed
-
- Fletcher H, McShane H. Tuberculosis vaccines: Current status and future prospects. Expert Opinion on Emerging Drugs. 2006;11:207–215. - PubMed
-
- de Jonge MI, Brosch R, Brodin P, et al. Tuberculosis: From genome to vaccine. Expert Review of Vaccines. 2005;4:541–551. - PubMed
-
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature Reviews Microbiology. 2005;3:656–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

