Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels
- PMID: 26315818
- PMCID: PMC4656112
- DOI: 10.1016/j.jconrel.2015.08.040
Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels
Abstract
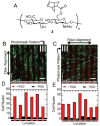
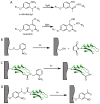
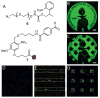
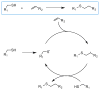
Hydrogels have emerged as promising scaffolds in regenerative medicine for the delivery of biomolecules to promote healing. However, increasing evidence suggests that the context that biomolecules are presented to cells (e.g., as soluble verses tethered signals) can influence their bioactivity. A common approach to deliver biomolecules in hydrogels involves physically entrapping them within the network, such that they diffuse out over time to the surrounding tissues. While simple and versatile, the release profiles in such system are highly dependent on the molecular weight of the entrapped molecule relative to the network structure, and it can be difficult to control the release of two different signals at independent rates. In some cases, supraphysiologically high loadings are used to achieve therapeutic local concentrations, but uncontrolled release can then cause deleterious off-target side effects. In vivo, many growth factors and cytokines are stored in the extracellular matrix (ECM) and released on demand as needed during development, growth, and wound healing. Thus, emerging strategies in biomaterial chemistry have focused on ways to tether or sequester biological signals and engineer these bioactive scaffolds to signal to delivered cells or endogenous cells. While many strategies exist to achieve tethering of peptides, protein, and small molecules, this review focuses on photochemical methods, and their usefulness as a mild reaction that proceeds with fast kinetics in aqueous solutions and at physiological conditions. Photo-click and photo-caging methods are particularly useful because one can direct light to specific regions of the hydrogel to achieve spatial patterning. Recent methods have even demonstrated reversible introduction of biomolecules to mimic the dynamic changes of native ECM, enabling researchers to explore how the spatial and dynamic context of biomolecular signals influences important cell functions. This review will highlight how two photochemical methods have led to important advances in the tissue regeneration community, namely the thiol-ene photo-click reaction for bioconjugation and photocleavage reactions that allow for the removal of protecting groups. Specific examples will be highlighted where these methodologies have been used to engineer hydrogels that control and direct cell function with the aim of inspiring their use in regenerative medicine.
Keywords: Hydrogel; Immobilization; Patterning; Photo-cage; Regenerative medicine; Thiol-ene.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures






References
-
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. - PubMed
-
- Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. - PubMed
-
- Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. - PubMed
-
- Mellott MB, Searcy K, Pishko MV. Release of protein from highly cross-linked hydrogels of poly (ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. - PubMed
-
- Lin CC, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv Drug Delivery Rev. 2006;58:1379–1408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

