Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1
- PMID: 26315932
- PMCID: PMC4666326
- DOI: 10.3324/haematol.2015.128777
Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1
Abstract
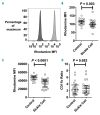
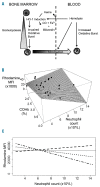
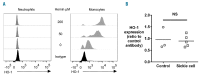
Sickle cell disease is a risk factor for invasive bacterial infections, and splenic dysfunction is believed to be the main underlying cause. We have previously shown that the liberation of heme in acute hemolysis can induce heme oxygenase-1 during granulopoiesis, impairing the ability of developing neutrophils to mount a bactericidal oxidative burst, and increasing susceptibility to bacterial infection. We hypothesized that this may also occur with the chronic hemolysis of sickle cell disease, potentially contributing to susceptibility to infections. We found that neutrophil oxidative burst activity was significantly lower in treatment-naïve children with sickle cell disease compared to age-, gender- and ethnicity-matched controls, whilst degranulation was similar. The defect in neutrophil oxidative burst was quantitatively related to both systemic heme oxygenase-1 activity (assessed by carboxyhemoglobin concentration) and neutrophil mobilization. A distinct population of heme oxygenase-1-expressing cells was present in the bone marrow of children with sickle cell disease, but not in healthy children, with a surface marker profile consistent with neutrophil progenitors (CD49d(Hi) CD24(Lo) CD15(Int) CD16(Int) CD11b(+/-)). Incubation of promyelocytic HL-60 cells with the heme oxygenase-1 substrate and inducer, hemin, demonstrated that heme oxygenase-1 induction during neutrophilic differentiation could reduce oxidative burst capacity. These findings indicate that impairment of neutrophil oxidative burst activity in sickle cell disease is associated with hemolysis and heme oxygenase-1 expression. Neutrophil dysfunction might contribute to risk of infection in sickle cell disease, and measurement of neutrophil oxidative burst might be used to identify patients at greatest risk of infection, who might benefit from enhanced prophylaxis.
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Ramakrishnan M, Moisi JC, Klugman KP, et al. Increased risk of invasive bacterial infections in African people with sickle-cell disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):329–337. - PubMed
-
- Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14(1):e2–e12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

