Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans
- PMID: 26315937
- PMCID: PMC4679498
- DOI: 10.1016/j.expneurol.2015.08.015
Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans
Abstract
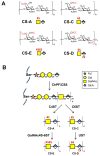
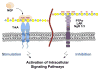
Chondroitin sulfate proteoglycans (CSPGs) play important roles in the developing and mature nervous system, where they guide axons, maintain stable connections, restrict synaptic plasticity, and prevent axon regeneration following CNS injury. The chondroitin sulfate glycosaminoglycan (CS GAG) chains that decorate CSPGs are essential for their functions. Through these sugar chains, CSPGs are able to bind and regulate the activity of a diverse range of proteins. CSPGs have been found both to promote and inhibit neuronal growth. They can promote neurite outgrowth by binding to various growth factors such as midkine (MK), pleiotrophin (PTN), brain-derived neurotrophic factor (BDNF) and other neurotrophin family members. CSPGs can also inhibit neuronal growth and limit plasticity by interacting with transmembrane receptors such as protein tyrosine phosphatase σ (PTPσ), leukocyte common antigen-related (LAR) receptor protein tyrosine phosphatase, and the Nogo receptors 1 and 3 (NgR1 and NgR3). These CS-protein interactions depend on specific sulfation patterns within the CS GAG chains, and accordingly, particular CS sulfation motifs are upregulated during development, in the mature nervous system, and in response to CNS injury. Thus, spatiotemporal regulation of CS GAG biosynthesis may provide an important mechanism to control the functions of CSPGs and to modulate intracellular signaling pathways. Here, we will discuss these sulfation-dependent processes and highlight how the CS sugars on CSPGs contribute to neuronal growth, axon guidance, and plasticity in the nervous system.
Keywords: Axon guidance; Axon regeneration; CSPG; CSPG receptor; Chondroitin sulfate (CS); Glycosaminoglycans; Neuronal growth; Neuronal injury; Plasticity; Proteoglycan.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Bao X, Nishimura S, Mikami T, Yamada S, Itoh N, Sugahara K. Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of L-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J Biol Chem. 2004;279:9765–9776. - PubMed
-
- Bao X, Mikami T, Yamada S, Faissner A, Muramatsu T, Sugahara K. Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J Biol Chem. 2005;280:9180–9191. - PubMed
-
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235:5–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

