In utero exposure to di-(2-ethylhexyl) phthalate induces testicular effects in neonatal rats that are antagonized by genistein cotreatment
- PMID: 26316063
- PMCID: PMC4711908
- DOI: 10.1095/biolreprod.115.129098
In utero exposure to di-(2-ethylhexyl) phthalate induces testicular effects in neonatal rats that are antagonized by genistein cotreatment
Abstract
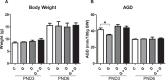
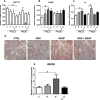
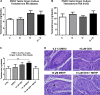
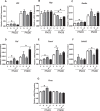
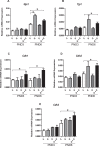
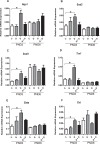
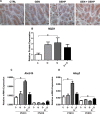
Fetal exposure to endocrine disruptors (EDs) is believed to predispose males to reproductive abnormalities. Although males are exposed to combinations of chemicals, few studies have evaluated the effects of ED mixtures at environmentally relevant doses. Our previous work showed that fetal exposure to a mixture of the phytoestrogen genistein (GEN) and the plasticizer di-(2-ethylhexyl) phthalate (DEHP) induced unique alterations in adult testis. In this follow-up study, we examined Postnatal Day 3 (PND3) and PND6 male offspring exposed from Gestational Day 14 to parturition to corn oil, 10mg/kg GEN, DEHP, or their combination, to gain insight into the early molecular events driving long-term alterations. DEHP stimulated the mRNA and protein expression of the steroidogenic enzyme HSD3B, uniquely at PND3. DEHP also increased the mRNA expression of Nestin, a Leydig progenitor/Sertoli cell marker, and markers of Sertoli cell (Wt1), gonocyte (Plzf, Foxo1), and proliferation (Pcna) at PND3, while these genes were unchanged by the mixture. Redox (Nqo1, Sod2, Sod3, Trx, Gst, Cat) and xenobiotic transporter (Abcb1b, Abcg2) gene expression was also increased by DEHP at PND3, while attenuated when combined with GEN, suggesting the involvement of cellular stress in short-term DEHP effects and a protective effect of GEN. The direct effects of GEN and mono-(2-ethylhexyl) phthalate, the principal bioactive metabolite of DEHP, on testis were investigated in PND3 organ cultures, showing a stimulatory effect of 10 μM mono-(2-ethylhexyl) phthalate on basal testosterone production that was normalized by GEN. These effects contrasted with previous reports of androgen suppression and decreased gene expression in perinatal rat testis by high DEHP doses, implying that neonatal effects are not predictive of adult effects. We propose that GEN, through an antioxidant action, normalizes reactive oxygen species-induced neonatal effects of DEHP. The notion that these EDs do not follow classical dose-response effects and involve different mechanisms of toxicity from perinatal ages to adulthood highlights the importance of assessing impacts across a range of doses and ages.
Keywords: Leydig; ROS; Sertoli; endocrine disruptor; gene expression; genistein; germ cells; gonadal function; mixture; phthalate; rodents (mice, guinea pigs, rats, voles); testis; toxicology.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures
References
-
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. - PubMed
-
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. - PubMed
-
- Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res a Clin Mol Teratol. 2010;88:910–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

