Cardiomyocytes induce macrophage receptor shedding to suppress phagocytosis
- PMID: 26316303
- PMCID: PMC4637185
- DOI: 10.1016/j.yjmcc.2015.08.009
Cardiomyocytes induce macrophage receptor shedding to suppress phagocytosis
Abstract
Background: Mobilization of the innate immune response to clear and metabolize necrotic and apoptotic cardiomyocytes is a prerequisite to heart repair after cardiac injury. Suboptimal kinetics of dying myocyte clearance leads to secondary necrosis, and in the case of the heart, increased potential for collateral loss of neighboring non-regenerative myocytes. Despite the importance of myocyte phagocytic clearance during heart repair, surprisingly little is known about its underlying cell and molecular biology.
Objective: To determine if phagocytic receptor MERTK is expressed in human hearts and to elucidate key sequential steps and phagocytosis efficiency of dying adult cardiomyocytes, by macrophages.
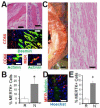
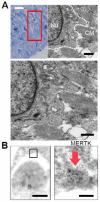
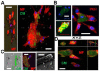
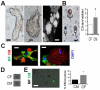
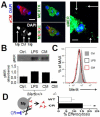
Results: In infarcted human hearts, expression profiles of the phagocytic receptor MER-tyrosine kinase (MERTK) mimicked that found in experimental ischemic mouse hearts. Electron micrographs of myocardium identified MERTK signal along macrophage phagocytic cups and Mertk-/- macrophages contained reduced digested myocyte debris after myocardial infarction. Ex vivo co-culture of primary macrophages and adult cardiomyocyte apoptotic bodies revealed reduced engulfment relative to resident cardiac fibroblasts. Inefficient clearance was not due to the larger size of myocyte apoptotic bodies, nor were other key steps preceding the formation of phagocytic synapses significantly affected; this included macrophage chemotaxis and direct binding of phagocytes to myocytes. Instead, suppressed phagocytosis was directly associated with myocyte-induced inactivation of MERTK, which was partially rescued by genetic deletion of a MERTK proteolytic susceptibility site.
Conclusion: Utilizing an ex vivo co-cultivation approach to model key cellular and molecular events found in vivo during infarction, cardiomyocyte phagocytosis was found to be inefficient, in part due to myocyte-induced shedding of macrophage MERTK. These findings warrant future studies to identify other cofactors of macrophage-cardiomyocyte cross-talk that contribute to cardiac pathophysiology.
Keywords: Acute myocardial infarction; Animal models of human disease; Cardiomyocyte; Phagocytosis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

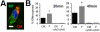

References
-
- Aderem A. How to eat something bigger than your head. Cell. 2002;110(1):5–8. - PubMed
-
- Camenisch TD, Koller BH, et al. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162(6):3498–3503. - PubMed
-
- Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101(Pt 4):907–913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

