Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury
- PMID: 26316358
- PMCID: PMC4674329
- DOI: 10.1016/j.nbd.2015.08.021
Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury
Abstract
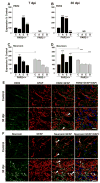
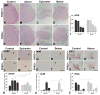
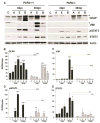
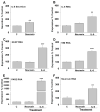
Inflammatory-astrogliosis exacerbates damage in the injured spinal cord and limits repair. Here we identify Protease Activated Receptor 2 (PAR2) as an essential regulator of these events with mice lacking the PAR2 gene showing greater improvements in motor coordination and strength after compression-spinal cord injury (SCI) compared to wild type littermates. Molecular profiling of the injury epicenter, and spinal segments above and below, demonstrated that mice lacking PAR2 had significantly attenuated elevations in key hallmarks of astrogliosis (glial fibrillary acidic protein (GFAP), vimentin and neurocan) and in expression of pro-inflammatory cytokines (interleukin-6 (IL-6), tumor necrosis factor (TNF) and interleukin-1 beta (IL-1β)). SCI in PAR2-/- mice was also accompanied by improved preservation of protein kinase C gamma (PKCγ)-immunopositive corticospinal axons and reductions in GFAP-immunoreactivity, expression of the pro-apoptotic marker BCL2-interacting mediator of cell death (BIM), and in signal transducer and activator of transcription 3 (STAT3). The potential mechanistic link between PAR2, STAT3 and astrogliosis was further investigated in primary astrocytes to reveal that the SCI-related serine protease, neurosin (kallikrein 6) promotes IL-6 secretion in a PAR2 and STAT3-dependent manner. Data point to a signaling circuit in primary astrocytes in which neurosin signaling at PAR2 promotes IL-6 secretion and canonical STAT3 signaling. IL-6 promotes expression of GFAP, vimentin, additional IL-6 and robust increases in both neurosin and PAR2, thereby driving the PAR2-signaling circuit forward. Given the significant reductions in astrogliosis and inflammation as well as superior neuromotor recovery observed in PAR2 knockout mice after SCI, we suggest that this receptor and its agonists represent new drug targets to foster neuromotor recovery.
Keywords: Astrogliosis; Cytokine; GPCR; Inflammation; Serine protease; Traumatic spinal cord injury.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Asher RA, Morgenstern DA, Moon LD, Fawcett JW. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res. 2001;132:611–619. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014;258:62–77. - PubMed
-
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

