The Impact of Different CD4 Cell-Count Monitoring and Switching Strategies on Mortality in HIV-Infected African Adults on Antiretroviral Therapy: An Application of Dynamic Marginal Structural Models
- PMID: 26316598
- PMCID: PMC4581589
- DOI: 10.1093/aje/kwv083
The Impact of Different CD4 Cell-Count Monitoring and Switching Strategies on Mortality in HIV-Infected African Adults on Antiretroviral Therapy: An Application of Dynamic Marginal Structural Models
Abstract
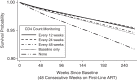
In Africa, antiretroviral therapy (ART) is delivered with limited laboratory monitoring, often none. In 2003-2004, investigators in the Development of Antiretroviral Therapy in Africa (DART) Trial randomized persons initiating ART in Uganda and Zimbabwe to either laboratory and clinical monitoring (LCM) or clinically driven monitoring (CDM). CD4 cell counts were measured every 12 weeks in both groups but were only returned to treating clinicians for management in the LCM group. Follow-up continued through 2008. In observational analyses, dynamic marginal structural models on pooled randomized groups were used to estimate survival under different monitoring-frequency and clinical/immunological switching strategies. Assumptions included no direct effect of randomized group on mortality or confounders and no unmeasured confounders which influenced treatment switch and mortality or treatment switch and time-dependent covariates. After 48 weeks of first-line ART, 2,946 individuals contributed 11,351 person-years of follow-up, 625 switches, and 179 deaths. The estimated survival probability after a further 240 weeks for post-48-week switch at the first CD4 cell count less than 100 cells/mm(3) or non-Candida World Health Organization stage 4 event (with CD4 count <250) was 0.96 (95% confidence interval (CI): 0.94, 0.97) with 12-weekly CD4 testing, 0.96 (95% CI: 0.95, 0.97) with 24-weekly CD4 testing, 0.95 (95% CI: 0.93, 0.96) with a single CD4 test at 48 weeks (baseline), and 0.92 (95% CI: 0.91, 0.94) with no CD4 testing. Comparing randomized groups by 48-week CD4 count, the mortality risk associated with CDM versus LCM was greater in persons with CD4 counts of <100 (hazard ratio = 2.4, 95% CI: 1.3, 4.3) than in those with CD4 counts of ≥100 (hazard ratio = 1.1, 95% CI: 0.8, 1.7; interaction P = 0.04). These findings support a benefit from identifying patients immunologically failing first-line ART at 48 weeks.
Keywords: Africa; HIV; antiretroviral therapy; drug switching; dynamic marginal structural models; monitoring.
© The Author 2015. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health.
Figures


References
-
- Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;3689534:505–510. - PubMed
-
- Hernán MA, Lanoy E, Costagliola D, et al. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;983:237–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

