A novel, long-acting glucagon-like peptide receptor-agonist: dulaglutide
- PMID: 26316788
- PMCID: PMC4541559
- DOI: 10.2147/DMSO.S34418
A novel, long-acting glucagon-like peptide receptor-agonist: dulaglutide
Abstract
Background: Dulaglutide is a new, long-acting glucagon-like peptide analogue in the treatment of type 2 diabetes. It is available in two doses, 0.75 and 1.5 mg, given by injection once weekly. This systematic review reports the effectiveness and safety of dulaglutide in type 2 diabetes in dual and triple therapy.
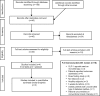
Methods: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, and conference abstracts were searched from 2005 to August 2014, and updated in January 2015. Company websites and references of included studies were checked for potentially relevant studies. European Medicines Agency and US Food and Drug Administration websites were searched.
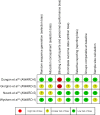
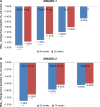
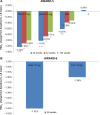
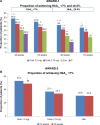
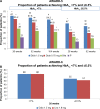
Results: Four trials were included. All were manufacturer-funded randomized controlled trials from the Assessment of Weekly Administration of Dulaglutide in Diabetes (AWARD) program. AWARD-1 compared dulaglutide 1.5 mg against exenatide 10 µg twice daily and placebo, AWARD-2 compared dulaglutide 0.75 and 1.5 mg against insulin glargine, AWARD-5 compared dulaglutide 0.75 and 1.5 mg against sitagliptin 100 mg and placebo, and AWARD-6 compared dulaglutide 1.5 mg against liraglutide 1.8 mg. The duration of follow-up in the trials ranged from 26 to 104 weeks. The primary outcome of all the included trials was change in HbA1c. At 26 weeks, greater HbA1c reductions were seen with dulaglutide than with twice daily exenatide (dulaglutide 1.5/0.75 mg: -1.5%/-1.3%; exe: 0.99%) and sitagliptin (1.5/0.75 mg -1.22%/-1.01%; sitagliptin: -0.6%). HbA1c change was greater with dulaglutide 1.5 mg (-1.08%) than with glargine (-0.63%), but not with dulaglutide 0.75 mg (-0.76%). Dulaglutide 1.5 mg was found to be noninferior to liraglutide 1.8 mg. More patients treated with dulaglutide achieved HbA1c targets of <7% and ≤6.5%. Reduction in weight was greater with dulaglutide than with sitagliptin and exenatide. Hypoglycemia was infrequent. The main adverse events were nausea, diarrhea, and vomiting.
Conclusion: Dulaglutide is effective in the treatment of patients with type 2 diabetes but we need long follow-up data for safety concerns.
Keywords: dulaglutide; effectiveness; glucagon-like peptide analogue; glycemic control; type 2 diabetes.
Figures


References
-
- Turner R, Cull C, Holman R. United Kingdom prospective diabetes study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124(1 Pt 2):136–145. - PubMed
-
- Internation diabetes federation (IDF) Diabetes Atlas. 6th ed. Brussels, Belgium: IDF; 2013. [Accessed December 1, 2014]. Available from: http://www.idf.org/diabetesatlas.
-
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. - PMC - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. - PubMed
-
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–2500. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

